Early warning of Aspergillus contamination in maize by gas chromatography-ion mobility spectrometry
- PMID: 39391609
- PMCID: PMC11464317
- DOI: 10.3389/fmicb.2024.1470115
Early warning of Aspergillus contamination in maize by gas chromatography-ion mobility spectrometry
Abstract
Introduction: As one of the main grain crops in China, maize is highly susceptible to Aspergillus infection during processing, storage and transportation due to high moisture at harvest, which results in the loss of quality. The aim of this study is to explore the early warning marker molecules when Aspergillus infects maize kernels.
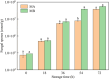
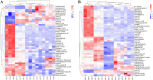
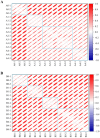
Methods: Firstly, strains MA and MB were isolated from moldy maize and identified by morphological characterization and 18S rRNA gene sequence analysis to be Aspergillus flavus (A. flavus) and Aspergillus niger (A. niger). Next, fresh maize was moldy by contaminated with strains MA and MB. The volatile organic compounds (VOCs) during the contamination process of two fungal strains were analyzed by gas chromatography-ion mobility spectrometry (GC-IMS). A total of 31 VOCs were detected in maize contaminated with strain MA, a total of 32 VOCs were detected in maize contaminated with strain MB, including confirmed monomers and dimers. Finally, heat maps and principal component analysis (PCA) showed that VOCs produced in different growth stages of Aspergillus had great differences. Combined with the results of GC-IMS, total fungal colony counts and fungal spores, it was concluded that the Aspergillus-contaminated maize was in the early stage of mold at 18 h.
Results: Therefore, the characteristic VOCs butan-2-one, ethyl acetate-D, Benzaldehyde, and pentan-2-one produced by maize at 18 h of storage can be used as early mildew biomarkers of Aspergillus infection in maize.
Discussion: This study provided effective marker molecules for the development of an early warning and monitoring system for the degree of maize mildew in granaries.
Keywords: Aspergillus flavus; Aspergillus niger; gas chromatography-ion mobility spectrometry (GC-IMS); maize; principal component analysis (PCA); volatile organic compounds (VOCs).
Copyright © 2024 Qin, Lv, Xiong, Qi, Li, Xin and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Arroyo-Manzanares N., Martín-Gómez A., Jurado-Campos N., Garrido-Delgado R., Arce C., Arce L. (2018). Target vs spectral fingerprint data analysis of Iberian ham samples for avoiding labelling fraud using headspace—gas chromatography-ion mobility spectrometry. Food Chem. 246, 65–73. doi: 10.1016/j.foodchem.2017.11.008, PMID: - DOI - PubMed
-
- Bocate K. P., Evangelista A. G., Luciano F. B. (2021). Garlic essential oil as an antifungal and anti-mycotoxin agent in stored corn. LWT 147:111600. doi: 10.1016/j.lwt.2021.111600 - DOI
-
- Caligiore-Gei P. F., Moratalla-López N., Poggi L. M., Alonso G. L. (2023). Isolation, identification, and determination of the virulence of the causal agents of corm rot of saffron (Crocus sativus L.) in Valle de Uco, Argentina. Plants 12:2717. doi: 10.3390/plants12142717, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

