A double-blinded randomized control trial to compare the duration of analgesia using morphine, clonidine, or dexmedetomidine as adjuvants to ropivacaine in caudal anesthesia in children undergoing infraumbilical surgeries
- PMID: 39391669
- PMCID: PMC11463927
- DOI: 10.4103/joacp.joacp_12_23
A double-blinded randomized control trial to compare the duration of analgesia using morphine, clonidine, or dexmedetomidine as adjuvants to ropivacaine in caudal anesthesia in children undergoing infraumbilical surgeries
Abstract
Background and aims: Adjuvants added to the caudal block prolong the duration of analgesia. In a developing country with economic constraints, the choice of an adjuvant will be the medication with a longer duration of analgesia, a favorable side-effect profile, and the least expensive option. We wished to study the duration of postoperative analgesia afforded by three adjuvants: morphine, clonidine, and dexmedetomidine, at doses wherein minimal or nil adverse effects would be attributed to the adjuvant. The primary objective of the current study is to compare the duration of postoperative analgesia with morphine, clonidine, or dexmedetomidine as adjuvants to 0.2% ropivacaine in a for caudal block, in children undergoing elective abdominal, urogenital, and lower limb surgeries. The secondary objectives are (a) to study the total analgesic requirement during the first 24 hours after surgery and (b) to compare the incidence of complications among the three groups.
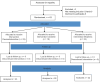
Material and methods: Sixty-three children aged 1-6 years, belonging to American Society of Anesthesia (ASA) physical status I, II, and scheduled to undergo elective infraumbilical surgeries, were enrolled in the study. The children were randomly assigned to one of three groups: Group D received a caudal block with dexmedetomidine 1 μg/kg, Group M received morphine 30 μg/kg, and Group C received clonidine 1.5 μg/kg. All groups also received 0.2% ropivacaine (1-1.25 ml/kg) as part of the caudal block. The duration of analgesia, total analgesic requirements during the first 24 hours after the surgery, and the incidence of complications in the three groups were monitored by a pain nurse who was blinded to the study allocation.
Results: The three groups were comparable with respect to age, sex, weight, and duration of surgery. The median time taken for the first rescue analgesic in the dexmedetomidine group was 380 minutes, in the clonidine group was 360 minutes, and in the morphine group was 405 minutes. Though the morphine group had a longer duration of analgesia, it was not statistically significant (P = 0.843). The total perioperative opioids used and side effects were similar among the three groups. There were no episodes of intraoperative bradycardia noted in Groups D, M, and C. However, one patient in Group D required treatment for bradycardia in the postanesthesia care unit. In terms of intraoperative hypotension, 10 patients (43.5%) in Group D, 5 patients (27.8%) in Group C, and 5 patients (22.7%) in Group M required treatment, but this difference was not statistically significant (P = 0.299). There was no significant difference observed in the time to awakening after the anesthesia among the three groups. Postoperative nausea and vomiting were noted in five patients (21.7%) in Group D, one patient (5.6%) in Group C, and four patients (18.2%) in Group M (P = 0.382). One patient in Group M had a sedation score of 5 and required 4 hours of supplemental oxygen via face mask in the ward. Additionally, one patient in Group D reported numbness in both feet lasting 12 hours with spontaneous resolution. While a significant number of patients in all three study groups experienced urinary retention, no patient reported pruritus in the ward.
Conclusion: Caudal administration of morphine, dexmedetomidine, and clonidine in children undergoing infraumbilical surgery resulted in an equivalent duration of analgesia.
Keywords: Analgesia; clonidine; developing country; dexmedetomidine; morphine; ropivacaine.
Copyright: © 2023 Journal of Anaesthesiology Clinical Pharmacology.
Conflict of interest statement
There are no conflicts of interest.
Figures

References
-
- de Beer DAH, Thomas ML. Caudal additives in children—solutions or problems? Br J Anaesth. 2003;90:487–98. - PubMed
-
- Ghai B, Makkar JK, Chari P, Rao KLN. Addition of midazolam to continuous postoperative epidural bupivacaine infusion reduces requirement for rescue analgesia in children undergoing upper abdominal and flank surgery. J Clin Anesth. 2009;21:113–9. - PubMed
-
- Breschan C, Krumpholz R, Likar R, Kraschl R, Schalk HV. Can a dose of 2 ?g/kg caudal clonidine cause respiratory depression in neonates? Pediatr Anesth. 1999;9:81–3. - PubMed
LinkOut - more resources
Full Text Sources
