A novel assay of excess plasma kallikrein-kinin system activation in hereditary angioedema
- PMID: 39391687
- PMCID: PMC11464748
- DOI: 10.3389/falgy.2024.1436855
A novel assay of excess plasma kallikrein-kinin system activation in hereditary angioedema
Erratum in
-
Corrigendum: A novel assay of excess plasma kallikrein-kinin system activation in hereditary angioedema.Front Allergy. 2025 Feb 14;6:1557356. doi: 10.3389/falgy.2025.1557356. eCollection 2025. Front Allergy. 2025. PMID: 40027353 Free PMC article.
-
Erratum: A novel assay of excess plasma kallikrein-kinin system activation in hereditary angioedema.Front Allergy. 2025 Apr 25;6:1608925. doi: 10.3389/falgy.2025.1608925. eCollection 2025. Front Allergy. 2025. PMID: 40351329 Free PMC article.
Abstract
Background: Cleaved high-molecular-weight kininogen (HKa) is a disease state biomarker of kallikrein-kinin system (KKS) activation in patients with hereditary angioedema due to C1 inhibitor deficiency (HAE-C1INH), the endogenous inhibitor of plasma kallikrein (PKa).
Objective: Develop an HKa-specific enzyme-linked immunosorbent assay (ELISA) to monitor KKS activation in the plasma of HAE-C1INH patients.
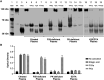
Methods: A novel HKa-specific antibody was discovered by antibody phage display and used as a capture reagent to develop an HKa-specific ELISA.
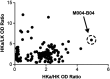
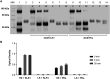
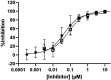
Results: Specific HKa detection following KKS activation was observed in plasma from healthy controls but not in prekallikrein-, high-molecular-weight kininogen-, or coagulation factor XII (FXII)-deficient plasma. HKa levels in plasma collected from HAE-C1INH patients in a disease quiescent state were higher than in plasma from healthy controls and increased further in HAE-C1INH plasma collected during an angioedema attack. The specificity of the assay for PKa-mediated HKa generation in minimally diluted plasma activated with exogenous FXIIa was demonstrated using a specific monoclonal antibody inhibitor (lanadelumab, IC50 = 0.044 µM).
Conclusions: An ELISA was developed for the specific and quantitative detection of HKa in human plasma to support HAE-C1INH drug development. Improved quantification of the HKa biomarker may facilitate further pathophysiologic insight into HAE-C1INH and other diseases mediated by a dysregulated KKS and may enable the design of highly potent inhibitors targeting this pathway.
Keywords: biomarkers; bradykinin; hereditary angioedema; phage display; plasma kallikrein.
© 2024 Sexton, Faucette, Rivera-Hernandez, Kenniston, Papaioannou, Cosic, Kopacz, Salmon, Beauchemin, Juethner and Yeung.
Conflict of interest statement
JC, KK, CB, and DY are employees of Takeda Development Center Americas, Inc., and hold stock/stock options in Takeda Pharmaceuticals Company Limited. SJ is an employee of Takeda Pharmaceuticals USA, Inc., and holds stock/stock options in Takeda Pharmaceuticals Company Limited. DS is an employee of Sexton Bio Consulting, LLC, and a former employee of Takeda Development Center Americas, Inc., and holds Takeda Pharmaceuticals Company Limited stock or stock options. NP, JK, and MR-H are former employees of Takeda Development Center Americas, Inc., and hold stock/stock options in Takeda Pharmaceuticals Company Limited. RF is a former employee of Shire, a Takeda company. GS is an employee of Charles River Laboratories. The authors declare that this study received funding from Takeda. The funder had the following involvement in the study: research and publication of this article.
Figures

References
LinkOut - more resources
Full Text Sources

