C9orf72 poly-PR forms anisotropic condensates causative of nuclear TDP-43 pathology
- PMID: 39391721
- PMCID: PMC11465050
- DOI: 10.1016/j.isci.2024.110937
C9orf72 poly-PR forms anisotropic condensates causative of nuclear TDP-43 pathology
Abstract
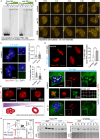
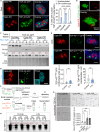
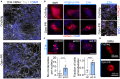
Proteinaceous inclusions formed by C9orf72-derived dipeptide-repeat (DPR) proteins are a histopathological hallmark in ∼50% of familial amyotrophic lateral sclerosis/frontotemporal dementia (ALS/FTD) cases. However, DPR aggregation/inclusion formation could not be efficiently recapitulated in cell models for four out of five DPRs. In this study, using optogenetics, we achieved chemical-free poly-PR condensation/aggregation in cultured cells including human motor neurons, with spatial and temporal control. Strikingly, nuclear poly-PR condensates had anisotropic, hollow-center appearance, resembling TDP-43 anisosomes, and their growth was limited by RNA. These condensates induced abnormal TDP-43 granulation in the nucleus without stress response activation. Cytoplasmic poly-PR aggregates forming under prolonged opto-stimulation were more persistent than its nuclear condensates, selectively sequestered TDP-43 in a demixed state and surrounded spontaneous stress granules. Thus, poly-PR condensation accompanied by nuclear TDP-43 dysfunction may constitute an early pathological event in C9-ALS/FTD. Anisosome-type condensates of disease-linked proteins may represent a common molecular species in neurodegenerative disease.
Keywords: Biochemistry; Cell biology; Molecular biology.
© 2024 The Author(s).
Conflict of interest statement
Authors declare no competing interests.
Figures



References
-
- Van Mossevelde S., van der Zee J., Cruts M., Van Broeckhoven C. Relationship between C9orf72 repeat size and clinical phenotype. Curr. Opin. Genet. Dev. 2017;44:117–124. - PubMed
-
- Ash P.E.A., Bieniek K.F., Gendron T.F., Caulfield T., Lin W.L., Dejesus-Hernandez M., van Blitterswijk M.M., Jansen-West K., Paul J.W., 3rd, Rademakers R., et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77:639–646. - PMC - PubMed
-
- Mori K., Weng S.M., Arzberger T., May S., Rentzsch K., Kremmer E., Schmid B., Kretzschmar H.A., Cruts M., Van Broeckhoven C., et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science. 2013;339:1335–1338. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

