GATA-4 overexpressing BMSC-derived exosomes suppress H/R-induced cardiomyocyte ferroptosis
- PMID: 39391723
- PMCID: PMC11466636
- DOI: 10.1016/j.isci.2024.110784
GATA-4 overexpressing BMSC-derived exosomes suppress H/R-induced cardiomyocyte ferroptosis
Abstract
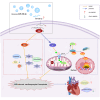
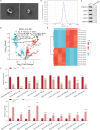
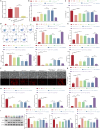
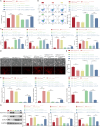
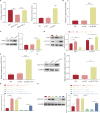
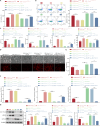
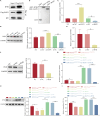
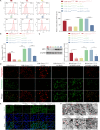
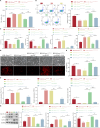
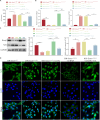
Bone marrow mesenchymal stem cell (BMSC)-derived exosomes overexpressing GATA-4 (Exosoe-GATA-4) can protect cardiac function. Mitochondrial permeability transition pore (mPTP) has a crucial role in ferroptosis. This study aimed to assess the mechanism of Exosoe-GATA-4 in myocardial ischemia/reperfusion (I/R) injury. Exos were successfully excreted, and 185 differential expression miRNAs were obtained using bioinformatics. The Exosoe-GATA-4 effectively suppressed hypoxia/reoxygenation (H/R)-induced cardiomyocytes' ferroptosis, while the effects were reversed by miR-330-3p inhibitor. miR-330-3p targeted negative regulated BAP1. The effects of miR-330-3p inhibitor were reversed by knock-down BAP1. Also, BAP1 reversed the effects of Exosoe-GATA-4 on H/R-induced cardiomyocytes' ferroptosis by downregulating SLC7A11. Mechanistically, BAP1 interacted with IP3R and increased cardiomyocytes' Ca2+ level, causing mPTP opening and mitochondrial dysfunction, promoting H/R-induced cardiomyocytes' ferroptosis. Moreover, hydrogen sulfide (H2S) content was increased and regulated the keap1/Nrf2 signaling pathway by Exosoe-GATA-4 treated. Exosoe-GATA-4 effectively suppresses H/R-induced cardiomyocytes' ferroptosis by upregulating miR-330-3p, which regulates the BAP1/SLC7A11/IP3R axis and inhibits mPTP opening.
Keywords: Cell biology; Molecular biology.
© 2024 The Author(s).
Conflict of interest statement
All authors declare no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

