Alternative splicing dysregulation across tissue and therapeutic approaches in a mouse model of myotonic dystrophy type 1
- PMID: 39391766
- PMCID: PMC11465180
- DOI: 10.1016/j.omtn.2024.102338
Alternative splicing dysregulation across tissue and therapeutic approaches in a mouse model of myotonic dystrophy type 1
Abstract
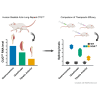
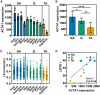
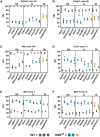
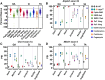
Myotonic dystrophy type 1 (DM1), the leading cause of adult-onset muscular dystrophy, is caused by a CTG repeat expansion. Expression of the repeat causes widespread alternative splicing (AS) defects and downstream pathogenesis, including significant skeletal muscle impacts. The HSA LR mouse model plays a significant role in therapeutic development. This mouse model features a transgene composed of approximately 220 interrupted CTG repeats, which results in skeletal muscle pathology that mirrors DM1. To better understand this model and the growing number of therapeutic approaches developed with it, we performed a meta-analysis of publicly available RNA sequencing data for AS changes across three widely examined skeletal muscles: quadriceps, gastrocnemius, and tibialis anterior. Our analysis demonstrated that transgene expression correlated with the extent of splicing dysregulation across these muscles from gastrocnemius (highest), quadriceps (medium), to tibialis anterior (lowest). We identified 95 splicing events consistently dysregulated across all examined datasets. Comparison of splicing rescue across seven therapeutic approaches showed a range of rescue across the 95 splicing events from the three muscle groups. This analysis contributes to our understanding of the HSA LR model and the growing number of therapeutic approaches currently in preclinical development for DM1.
Keywords: DM1; HSALR; MT: Bioinformatics; RNA sequencing; RNA-seq; alternative splicing; therapeutics.
© 2024 The Author(s).
Conflict of interest statement
J.A.B. serves on the Scientific Advisory Committee for the Myotonic Dystrophy Foundation, has consulted or currently consults for Entrada Therapeutics, Juvena Therapeutics, Kate Therapeutics, D.E. Shaw Research, Dyne Therapeutics, Syros Pharmaceuticals, and Wayfinder Biosciences, and has received research funding from Agios Pharmaceuticals, Biomarin Pharmaceuticals, PepGen, Syros Pharmaceuticals, and Vertex Pharmaceuticals. E.T.W. is a co-founder and consultant to Kate Therapeutics. J.A.B. has received licensing royalties from the University of Florida. J.A.B. and J.D.C. are co-founders and have a financial interest in Repeat RNA Therapeutics Inc. J.D.C. is a part-time employee of the Center for NeuroGenetics at the University of Florida.
Figures
References
-
- Brook J.D., McCurrach M.E., Harley H.G., Buckler A.J., Church D., Aburatani H., Hunter K., Stanton V.P., Thirion J.P., Hudson T., et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell. 1992;68:799–808. doi: 10.1016/0092-8674(92)90154-5. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

