Prognostic value of [18F]FDG PET/CT in metastatic hormone-sensitive prostate cancer at initial diagnosis: a retrospective cohort study
- PMID: 39392016
- PMCID: PMC11485890
- DOI: 10.1080/07853890.2024.2411017
Prognostic value of [18F]FDG PET/CT in metastatic hormone-sensitive prostate cancer at initial diagnosis: a retrospective cohort study
Abstract
Introduction: This retrospective study aimed to evaluate the prognostic value of [18F]FDG parameters in patients with visceral and bone metastatic hormone-sensitive prostate cancer (mHSPC).
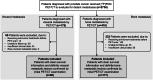
Patients and methods: This analysis included the mHSPC patients who underwent [18F]FDG PET/CT at the initial diagnosis. Baseline characteristics were analyzed, and the uptake of [18F]FDG was quantified using SUVmax. Kaplan-Meier and Cox proportional hazard regression analysis were employed to evaluate the correlation between SUVmax and patient survival.
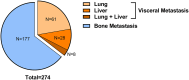
Results: Among the 267 patients enrolled, 90 (33.7%) presented with visceral metastases and 177 (66.3%) had bone metastases. The median follow-up for the visceral metastasis group was 35.5 months (IQR 26-53.8 months). The median overall survival for patients with lung, liver, or both metastases were 30, 21 and 17 months, respectively. Patients exhibiting higher [18F]FDG uptake in metastatic lesions experienced shorter overall survival (OS) in comparison to those with lower [18F]FDG uptake, both in the visceral metastases group (17 vs. 31 months, p = 0.002) and the bone metastases group (27.5 vs. 34.5 months, p < 0.001). Cox regression analysis further revealed that increased [18F]FDG uptake in metastatic lesions emerged as a significant risk factor in both OS and progression-free survival (PFS). In contrast, the variability in [18F]FDG uptake in primary lesions did not provide a reliable indicator for predicting prognosis.
Conclusions: In mHSPC patients, higher [18F]FDG uptake in metastatic lesions indicates shorter survival and increased risk of disease progression. The [18F]FDG SUVmax in primary tumors did not show significant prognostic value. Our study underscores the unique prognostic potential of [18F]FDG PET/CT in mHSPC patients, highlighting its importance in the management of both bone and visceral metastases.
Keywords: 18F-fludeoxyglucose; Metastatic hormone-sensitive prostate cancer; bone metastasis; prognosis; visceral metastasis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




Similar articles
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
-
[Clinical Value of Dual Tracer PET Imaging With 68Ga-PSMA and 18F-FDG in Patients With Metastatic Prostate Cancer].Sichuan Da Xue Xue Bao Yi Xue Ban. 2024 Sep 20;55(5):1063-1070. doi: 10.12182/20240960201. Sichuan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 39507973 Free PMC article. Chinese.
-
Prognostic value of quantitative fluorodeoxyglucose measurements in newly diagnosed metastatic breast cancer.Cancer Med. 2013 Oct;2(5):725-33. doi: 10.1002/cam4.119. Epub 2013 Sep 12. Cancer Med. 2013. PMID: 24403238 Free PMC article.
-
Prognostic Value of 18F-FDG PET/CT in a Large Cohort of Patients with Advanced Metastatic Neuroendocrine Neoplasms Treated with Peptide Receptor Radionuclide Therapy.J Nucl Med. 2020 Nov;61(11):1560-1569. doi: 10.2967/jnumed.119.241414. Epub 2020 Mar 13. J Nucl Med. 2020. PMID: 32169914
-
Prospective Study of Serial 18F-FDG PET and 18F-Fluoride PET to Predict Time to Skeletal-Related Events, Time to Progression, and Survival in Patients with Bone-Dominant Metastatic Breast Cancer.J Nucl Med. 2018 Dec;59(12):1823-1830. doi: 10.2967/jnumed.118.211102. Epub 2018 May 10. J Nucl Med. 2018. PMID: 29748233 Free PMC article.
References
-
- Leyh-Bannurah SR, Karakiewicz PI, Pompe RS, et al. . Inverse stage migration patterns in North American patients undergoing local prostate cancer treatment: a contemporary population-based update in light of the 2012 USPSTF recommendations. World J Urol. 2019;37(3):469–479. doi: 10.1007/s00345-018-2396-2. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
