Making in vitro conditions more reflective of in vivo conditions for research on the teleost gastrointestinal tract
- PMID: 39392112
- PMCID: PMC11529878
- DOI: 10.1242/jeb.246440
Making in vitro conditions more reflective of in vivo conditions for research on the teleost gastrointestinal tract
Abstract
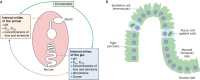
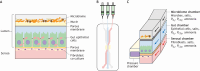
To date, the majority of in vitro or ex vivo fish gastrointestinal research has been conducted under unrealistic conditions. In a living fish, ionic conditions, as well as levels of ammonia, pH, HCO3- and PCO2 differ considerably between the different regions of the gastrointestinal tract. These factors also differ from those of the saline often used in gut research. Furthermore, the oxygen gradient from the serosa to the gut lumen is rarely considered: in contrast to the serosa, the lumen is a hypoxic/anoxic environment. In addition, the gut microbiome plays a significant role in gut physiology, increasing the complexity of the in vivo gut, but replicating the microbial community for in vitro studies is exceptionally difficult. However, there are ways in which we can begin to overcome these challenges. Firstly, the luminal chemistry and PO2 in each gut compartment must be carefully considered. Secondly, although microbiological culture techniques are improving, we must learn how to maintain the microbiome diversity seen in vivo. Finally, for ex vivo studies, developing mucosal (luminal) solutions that more closely mimic the in vivo conditions will better replicate physiological processes. Within the field of mammalian gut physiology, great advances in 'gut-on-chip' devices are providing the tools to better replicate in vivo conditions; adopting and adapting this technology may assist in fish gut research initiatives. This Commentary aims to make fish gut physiologists aware of the various issues in replicating the in vivo conditions and identifies solutions as well as those areas that require further improvement.
Keywords: Chyme; Gut microbiome; Intestine; Mucosal chemistry; Stomach.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures
Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Carbon dioxide detection for diagnosis of inadvertent respiratory tract placement of enterogastric tubes in children.Cochrane Database Syst Rev. 2025 Feb 19;2(2):CD011196. doi: 10.1002/14651858.CD011196.pub2. Cochrane Database Syst Rev. 2025. PMID: 39968844
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
Cited by
-
Osmoregulatory contributions of the corticotropin-releasing factor system in the intestine of Atlantic salmon.J Exp Biol. 2025 Jul 15;228(14):jeb250052. doi: 10.1242/jeb.250052. Epub 2025 May 15. J Exp Biol. 2025. PMID: 40123464 Free PMC article.
References
-
- Bakke, A., Glover, C. and Krogdahl, Å. (2011). Feeding, digestion, and absorption of nutrients. In Fish Physiology: Multifunctional Gut (ed. Grosell M., Farrell A. P. and Brauner C. J.), pp. 57-111. Elsevier Inc.
-
- Bakke-Mckellep, A. M., Nordrum, S., Krogdahl, Å. and Buddington, R. K. (2000). Absorption of glucose, amino acids, and dipeptides by the intestines of Atlantic salmon (Salmo salar L.). Fish Physiol.Biochem. 22, 33-44. 10.1023/A:1007872929847 - DOI
-
- Bols, N. C., Barlian, A., Chirino-Trejo, M., Caldwell, S. J., Goegan, P. and Lee, L. E. J. (1994a). Development of a cell line from primary cultures of rainbow trout, Oncorhynchus mykiss (Walbaum), gills. J. Fish Dis. 17, 601-611. 10.1111/j.1365-2761.1994.tb00258.x - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

