Personalized Intervention Strategy Based on a Risk Score Generated From Subcutaneous Insertable Cardiac Monitor: Results From Phase 1 of ALLEVIATE-HF
- PMID: 39392161
- PMCID: PMC11935577
- DOI: 10.1161/JAHA.124.035501
Personalized Intervention Strategy Based on a Risk Score Generated From Subcutaneous Insertable Cardiac Monitor: Results From Phase 1 of ALLEVIATE-HF
Abstract
Background: Diagnostic variables from insertable cardiac monitors may be useful in identifying patients at increased risk of heart failure (HF) events. High-risk alerts must be coupled with interventions to improve outcomes. We aim to assess the safety of a predefined protocolized intervention pathway activated by insertable cardiac monitor high-risk alerts.
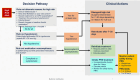
Methods and results: ALLEVIATE-HF (Algorithm Using LINQ Sensors for Evaluation and Treatment of Heart Failure) Phase 1 was a randomized interventional study enrolling patients with New York Heart Association class II/III and a recent HF event. A HF risk score based on insertable cardiac monitor diagnostics, including impedance, respiration rate, atrial fibrillation burden, heart rate during atrial fibrillation, heart rate variability, and activity duration, was calculated. A protocolized intervention pathway was activated when high-risk scores were detected that involved physician-prescribed nurse-implemented uptitration of diuretic for 4 days, unless safety rule-out conditions were met. Interventions could be repeated if high-risk scores persisted and did not require worsening symptoms. In total, 59 patients were randomized (mean age 68.2±11.8 years; 59.3% male); 67.8% with ejection fraction ≥50%. The mean follow-up was 11.8±8.1 months. Overall, 146 high-risk scores were recorded in 33 patients and 118 interventions occurred in 75 (51.4%) high-risk alerts that did not meet safety rule-out criteria. There were no serious adverse events and 13 adverse events related to interventions. In patients with symptoms at intervention initiation, symptoms resolved in 37 interventions (80%) and worsened in 8 (17%). In asymptomatic patients, symptoms developed in 3 interventions (7%).
Conclusions: A personalized medication intervention based on insertable cardiac monitor risk score can be safely instituted in patients with HF, irrespective of symptoms.
Registration: URL: https://www.clinicaltrials.gov; Unique Identifier: NCT04452149.
Keywords: heart failure; protocolized intervention pathway; remote monitoring; risk metric.
Figures

Comment in
-
Personalized Heart Failure Management: Bridging Technology and Care.J Am Heart Assoc. 2024 Oct 15;13(20):e037648. doi: 10.1161/JAHA.124.037648. Epub 2024 Oct 11. J Am Heart Assoc. 2024. PMID: 39392162 Free PMC article. No abstract available.
References
-
- Rogers JD, Sanders P, Piorkowski C, Sohail MR, Anand R, Crossen K, Khairallah FS, Kaplon RE, Stromberg K, Kowal RC. In‐office insertion of a miniaturized insertable cardiac monitor: results from the Reveal LINQ In‐Office 2 randomized study. Heart Rhythm. 2017;14:218–224. doi: 10.1016/j.hrthm.2016.11.001 - DOI - PubMed
-
- Beinart SC, Natale A, Verma A, Amin A, Kasner S, Diener HC, Del Greco M, Wilkoff BL, Pouliot E, Franco N, et al. Real‐world comparison of in‐hospital Reveal LINQ insertable cardiac monitor insertion inside and outside of the cardiac catheterization or electrophysiology laboratory. Am Heart J. 2019;207:76–82. doi: 10.1016/j.ahj.2018.10.002 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

