Thermal Denaturation of Fresh Frozen Tissue Enhances Mass Spectrometry Detection of Peptides
- PMID: 39392310
- PMCID: PMC11503521
- DOI: 10.1021/acs.analchem.4c03625
Thermal Denaturation of Fresh Frozen Tissue Enhances Mass Spectrometry Detection of Peptides
Abstract
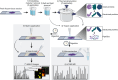
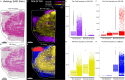
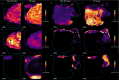
Thermal denaturation (TD), known as antigen retrieval, heats tissue samples in a buffered solution to expose protein epitopes. Thermal denaturation of formalin-fixed paraffin-embedded samples enhances on-tissue tryptic digestion, increasing peptide detection using matrix-assisted laser desorption ionization imaging mass spectrometry (MALDI IMS). We investigated the tissue-dependent effects of TD on peptide MALDI IMS and liquid chromatography-tandem mass spectrometry signal in unfixed, frozen human colon, ovary, and pancreas tissue. In a triplicate experiment using time-of-flight, orbitrap, and Fourier-transform ion cyclotron resonance mass spectrometry platforms, we found that TD had a tissue-dependent effect on peptide signal, resulting in a (22.5%) improvement in peptide detection from the colon, a (73.3%) improvement in ovary tissue, and a (96.6%) improvement in pancreas tissue. Biochemical analysis of identified peptides shows that TD facilitates identification of hydrophobic peptides.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

