An anti-Shiga toxin VHH nanobody multimer protects mice against fatal toxicosis when administered intramuscularly as repRNA
- PMID: 39392311
- PMCID: PMC11556087
- DOI: 10.1128/iai.00239-24
An anti-Shiga toxin VHH nanobody multimer protects mice against fatal toxicosis when administered intramuscularly as repRNA
Abstract
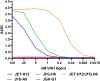
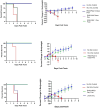
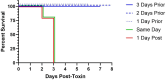
Hemolytic uremic syndrome (HUS) is a systemic sequelae from gastrointestinal infection with Shiga toxin (Stx) producing Escherichia coli (STEC) that can result in acute kidney injury, lasting renal disease, and death. Despite a window for intervention between hemorrhagic diarrhea and onset of HUS, no specific therapies exist to prevent or treat HUS following STEC infection. Furthermore, there is no way to predict which patients with STEC will develop HUS or any rapid way to determine which Stx variant is present. To address this, we have broadened the therpay to neutralize additional toxin variants. It contains a multimer of nanobodies derived from camelid heavy chain antibody fragments (VHHs). An improved
Keywords: E. coli O157; Shiga toxins; Stx mouse toxicity model; Stx treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Brandal LT, Wester AL, Lange H, Løbersli I, Lindstedt BA, Vold L, Kapperud G. 2015. Shiga toxin-producing Escherichia coli infections in Norway, 1992-2012: characterization of isolates and identification of risk factors for haemolytic uremic syndrome. BMC Infect Dis 15:324. doi: 10.1186/s12879-015-1017-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

