Unveiling the impact of Leptospira TolC efflux protein on host tissue adherence, complement evasion, and diagnostic potential
- PMID: 39392312
- PMCID: PMC11556070
- DOI: 10.1128/iai.00419-24
Unveiling the impact of Leptospira TolC efflux protein on host tissue adherence, complement evasion, and diagnostic potential
Abstract
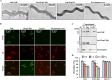
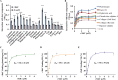
The TolC family protein of Leptospira is a type I outer membrane efflux protein. Phylogenetic analysis revealed significant sequence conservation among pathogenic Leptospira species (83%-98% identity) compared with intermediate and saprophytic species. Structural modeling indicated a composition of six β-strands and 10 α-helices arranged in two repeats, resembling bacterial outer membrane efflux proteins. Recombinant TolC (rTolC), expressed in a heterologous host and purified via Ni-NTA chromatography, maintained its secondary structural integrity, as verified by circular dichroism spectroscopy. Polyclonal antibodies against rTolC detected native TolC expression in pathogenic Leptospira but not in nonpathogenic ones. Immunoassays and detergent fractionation assays indicated surface localization of TolC. The rTolC's recognition by sera from leptospirosis-infected hosts across species suggests its utility as a diagnostic marker. Notably, rTolC demonstrated binding affinity for various extracellular matrix components, including collagen and chondroitin sulfate A, as well as plasma proteins such as factor H, C3b, and plasminogen, indicating potential roles in tissue adhesion and immune evasion. Functional assays demonstrated that rTolC-bound FH retained cofactor activity for C3b cleavage, highlighting TolC's role in complement regulation. The rTolC protein inhibited both the alternative and the classical pathway-mediated membrane attack complex (MAC) deposition in vitro. Blocking surface-expressed TolC on leptospires using specific antibodies reduced FH acquisition by Leptospira and increased MAC deposition on the spirochete. These findings indicate that TolC contributes to leptospiral virulence by promoting host tissue colonization and evading the immune response, presenting it as a potential target for diagnostic and therapeutic strategies.
Keywords: C3b; ECM; Leptospira; MAC; OMP; TolC; complement; efflux protein; factor H.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM, Peru-United States Leptospirosis Consortium . 2003. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis 3:757–771. doi: 10.1016/s1473-3099(03)00830-2 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

