Ferric Iron/Shikonin Nanoparticle-Embedded Hydrogels with Robust Adhesion and Healing Functions for Treating Oral Ulcers in Diabetes
- PMID: 39392368
- PMCID: PMC11615794
- DOI: 10.1002/advs.202405463
Ferric Iron/Shikonin Nanoparticle-Embedded Hydrogels with Robust Adhesion and Healing Functions for Treating Oral Ulcers in Diabetes
Abstract
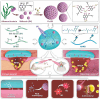
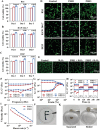
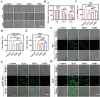
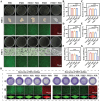
Oral ulcers can be addressed using various biomaterials designed to deliver medications or cytokines. Nevertheless, the effectiveness of these substances is frequently limited in many patients due to poor adherence, short retention time in the mouth, and less-than-optimal drug efficacy. In this study, a new hydrogel patch (FSH3) made of a silk fibroin/hyaluronic acid matrix with light-sensitive adhesive qualities infused with ferric iron/shikonin nanoparticles to enhance healing effects is presented. Initially, this hydrogel forms an adhesive barrier over mucosal lesions through a straightforward local injection, solidifying when exposed to UV light. Subsequently, FSH3 demonstrates superior reactive oxygen species elimination and near-infrared photothermal bactericidal activity. These characteristics support bacterial elimination and regulate oxidative levels, promoting a wound's progression from inflammation to tissue regeneration. In a diabetic rat model mimicking oral ulcers, FSH3 significantly speeds up healing by adjusting the inflammatory environment of the injured tissue, maintaining balance in oral microbiota, and promoting faster re-epithelialization. Overall, the light-sensitive FSH3 hydrogel shows potential for rapid wound recovery and may transform therapeutic methods for managing oral ulcers in diabetes.
Keywords: diabetes; hydrogels; oral ulcers; shikonin, tissue adhesives.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Wang Y., Pan Z., Cui J., Zhang X., Li D., Sun H., Yang B., Li Y., Acta Biomater. 2024, 178, 68. - PubMed
-
- Xiang Y., Qi X., Cai E., Zhang C., Wang J., Lan Y., Deng H., Shen J., Hu R., Chem. Eng. J. 2023, 460, 141852.
-
- Cai E., Qi X., Shi Y., Ge X., Xiang Y., Xu H., Li Y., Lan Y., Shen J., Hu R., Deng H., Chem. Eng. J. 2024, 488, 150372.
MeSH terms
Substances
Grants and funding
- LQ22E030011/Zhejiang Provincial Natural Science Foundation of China
- LR23C100001/Zhejiang Provincial Natural Science Foundation for Distinguished Young Scholar
- 82171144/National Natural Science Foundation of China
- 21977081/National Natural Science Foundation of China
- 82372551/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
