NK Cell and Monocyte Dysfunction in Multisystem Inflammatory Syndrome in Children
- PMID: 39392378
- PMCID: PMC11533154
- DOI: 10.4049/jimmunol.2400395
NK Cell and Monocyte Dysfunction in Multisystem Inflammatory Syndrome in Children
Abstract
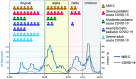
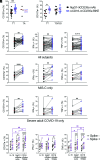
Multisystem inflammatory syndrome in children (MIS-C) is a severe complication of SARS-CoV-2 infection characterized by multiorgan involvement and inflammation. Testing of cellular function ex vivo to understand the aberrant immune response in MIS-C is limited. Despite strong Ab production in MIS-C, SARS-CoV-2 nucleic acid testing can remain positive for 4-6 wk postinfection. Therefore, we hypothesized that dysfunctional cell-mediated Ab responses downstream of Ab production may be responsible for delayed clearance of viral products in MIS-C. In MIS-C, monocytes were hyperfunctional for phagocytosis and cytokine production, whereas NK cells were hypofunctional for both killing and cytokine production. The decreased NK cell cytotoxicity correlated with an NK exhaustion marker signature and systemic IL-6 levels. Potentially providing a therapeutic option, cellular engagers of CD16 and SARS-CoV-2 proteins were found to rescue NK cell function in vitro. Taken together, our results reveal dysregulation in Ab-mediated cellular responses of myeloid and NK cells that likely contribute to the immune pathology of this disease.
Copyright © 2024 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures




References
-
- Vogel, T. P., Top K. A., Karatzios C., Hilmers D. C., Tapia L. I., Moceri P., Giovannini-Chami L., Wood N., Chandler R. E., Klein N. P., et al. 2021. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 39: 3037–3049. - PMC - PubMed
-
- Whittaker, E., Bamford A., Kenny J., Kaforou M., Jones C. E., Shah P., Ramnarayan P., Fraisse A., Miller O., Davies P., et al.; PIMS-TS Study Group and EUCLIDS and PERFORM Consortia . 2020. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 324: 259–269. - PMC - PubMed
-
- Feldstein, L. R., Rose E. B., Horwitz S. M., Collins J. P., Newhams M. M., Son M. B. F., Newburger J. W., Kleinman L. C., Heidemann S. M., Martin A. A., et al.; CDC COVID-19 Response Team . 2020. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 383: 334–346. - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
- T32AI007313-34A1/HHS | NIH | NIAID | Division of Microbiology and Infectious Diseases (DMID)
- T32 AI007313/AI/NIAID NIH HHS/United States
- UL1 TR002494/TR/NCATS NIH HHS/United States
- KL2TR002492/HHS | NIH | National Center for Advancing Translational Sciences (NCATS)
- KL2 TR002492/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

