Alzheimer's Disease: Exploring the Landscape of Cognitive Decline
- PMID: 39392435
- PMCID: PMC11587518
- DOI: 10.1021/acschemneuro.4c00339
Alzheimer's Disease: Exploring the Landscape of Cognitive Decline
Abstract
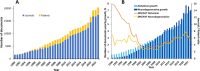
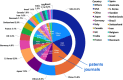
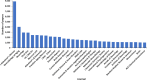
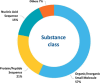
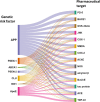
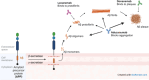
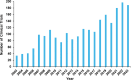
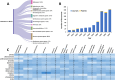
Alzheimer's disease (AD) is a progressive neurodegenerative disorder characterized by cognitive decline, memory loss, and impaired daily functioning. The pathology of AD is marked by the accumulation of amyloid beta plaques and tau protein tangles in the brain, along with neuroinflammation and synaptic dysfunction. Genetic factors, such as mutations in APP, PSEN1, and PSEN2 genes, as well as the APOE ε4 allele, contribute to increased risk of acquiring AD. Currently available treatments provide symptomatic relief but do not halt disease progression. Research efforts are focused on developing disease-modifying therapies that target the underlying pathological mechanisms of AD. Advances in identification and validation of reliable biomarkers for AD hold great promise for enhancing early diagnosis, monitoring disease progression, and assessing treatment response in clinical practice in effort to alleviate the burden of this devastating disease. In this paper, we analyze data from the CAS Content Collection to summarize the research progress in Alzheimer's disease. We examine the publication landscape in effort to provide insights into current knowledge advances and developments. We also review the most discussed and emerging concepts and assess the strategies to combat the disease. We explore the genetic risk factors, pharmacological targets, and comorbid diseases. Finally, we inspect clinical applications of products against AD with their development pipelines and efforts for drug repurposing. The objective of this review is to provide a broad overview of the evolving landscape of current knowledge regarding AD, to outline challenges, and to evaluate growth opportunities to further efforts in combating the disease.
Keywords: Alzheimer’s disease; aging; amyloid beta plaques; biomarker; pathogenesis; protein aggregation; tau protein tangles.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Veljkovic E., Xia W., Phillips B., Wong E. T., Ho J., Oviedo A., Hoeng J., Peitsch M.. Chapter 2 - Alzheimer’s Disease. In Nicotine and Other Tobacco Compounds in Neurodegenerative and Psychiatric Diseases; Veljkovic E., Xia W., Phillips B., Wong E. T., Ho J., Oviedo A., Hoeng J., Peitsch M., Eds.; Academic Press, 2018; pp 13–23.
-
- Savonenko A. V., Melnikova T., Li T., Price D. L., Wong P. C.. Chapter 21 - Alzheimer Disease. In Neurobiology of Brain Disorders; Zigmond M. J., Rowland L. P., Coyle J. T., Eds.; Academic Press: San Diego, 2015; pp 321–338.
-
- Budson A. E., Solomon P. R.. Chapter 3 - Alzheimer’s disease. In Memory Loss; Budson A. E., Solomon P. R., Eds.; W.B. Saunders: Edinburgh, 2011; pp 43–70.
-
See Chapter 2 for additional information.
-
- Boughey J. G. F., Graff-Radford N. R.. CHAPTER 65 - ALZHEIMER’S DISEASE. In Neurology and Clinical Neuroscience; Schapira A. H. V., Byrne E., DiMauro S., Frackowiak R. S. J., Johnson R. T., Mizuno Y., Samuels M. A., Silberstein S. D., Wszolek Z. K., Eds.; Mosby: Philadelphia, 2007; pp 846–858.
-
- Murray M. J., Murray C. F.. Chapter 118 - Alzheimer’s Disease. In Complications in Anesthesia (Second ed.); Atlee J. L., Ed.; W.B. Saunders: Philadelphia, 2007; pp 493–495.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

