Preclinical evaluation of 225Ac-labeled minigastrin analog DOTA-CCK-66 for Targeted Alpha Therapy
- PMID: 39392495
- PMCID: PMC11732879
- DOI: 10.1007/s00259-024-06927-z
Preclinical evaluation of 225Ac-labeled minigastrin analog DOTA-CCK-66 for Targeted Alpha Therapy
Abstract
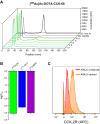
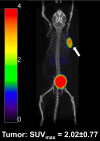
The recently developed metabolically more stable minigastrin derivative, DOTA-CCK-66, displayed promising preclinical data when labeled either with 68Ga or 177Lu. First positron emission tomography/computed tomography (PET/CT) imaging using [68Ga]Ga-DOTA-CCK-66 in two patients suffering from medullary thyroid carcinoma (MTC) displayed a favorable biodistribution profile. Here, we aim to investigate the therapeutic potential of [225Ac]Ac-DOTA-CCK-66 as a targeted α-therapy (TAT) agent in a comparative treatment study of [177Lu]Lu- versus [225Ac]Ac-DOTA-CCK-66.
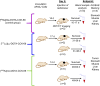
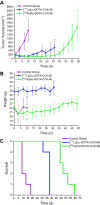
Methods: Treatment studies were performed (3 groups, n = 5, AR42J tumor-bearing 394-NOD SCID mice). Control group animals were injected with [68Ga]Ga-DOTA-CCK-66 (1.1 MBq, PET/CT imaging), while treatment group animals received a single dose of either [177Lu]Lu-DOTA-CCK-66 (37 MBq, radioligand therapy (RLT)) or [225Ac]Ac-DOTA-CCK-66 (37 kBq, TAT). All animals' tumor volume and body weight were monitored twice a week until end-point criteria were reached. Blood samples were evaluated (VetScan VS2, Abaxis) once mice were sacrificed.
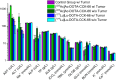
Results: Upon treatment, an initial decline in tumor volume, followed by a significantly delayed tumor growth of treated cohorts, was observed. Mean survival of 177Lu- as well as 225Ac-treated animals was increased by 3- (37 ± 3 d) and 4.5-fold (54 ± 6 d), respectively, when compared to non-treated animals (12 ± 3 d). Blood sample analysis did not indicate toxic side effects to the liver, kidney, or stomach upon 177Lu and 225Ac-treatment.
Conclusion: We demonstrated a substantial therapeutic efficacy of 177Lu- and 225Ac-labeled DOTA-CCK-66. As expected, treatment with the latter resulted in the highest mean survival rates. These results indicate a high therapeutic potential of 225Ac-labeled DOTA-CCK-66 for TAT in MTC patient management.
Keywords: Actinium-225; CCK-2R; MTC; Targeted Alpha Therapy (TAT).
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: All animal studies were approved by the UCLA Institutional Animal Care and Use Committee, known as the Chancellor’s Animal Research Committee (ARC; # 2005–090). The institutional guidelines for the care and use of animals were strictly followed. Mice were housed under pathogen-free conditions. Water and food were provided ad libitum. 394-NOD SCID mice were injected subcutaneously into the shoulder region. Tumor growth was monitored by CT. Animals were sacrificed upon reaching any of the termination criteria specified in the ARC protocol, including but not limited to apathy, ulceration, severe weight loss, or other signs of deteriorating condition. The study was carried out in compliance with the ARRIVE guidelines. This article does not contain any studies with human participants. Disclosure: A patent application on CCK-2R-targeted ligands, including DOTA-CCK-66, has been filed, with NH, CL, and TG as co-inventors. No other potential conflict of interest relevant to this article was reported.
Figures

References
-
- Delpassand ES, Tworowska I, Esfandiari R, Torgue J, Hurt J, Shafie A, Núñez R. Targeted α-Emitter therapy with 212Pb-DOTAMTATE for the treatment of metastatic SSTR-expressing neuroendocrine tumors: first-in-humans dose-escalation clinical trial. J Nucl Med. 2022;63:1326–33. 10.2967/jnumed.121.263230. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

