Diagnostic utility of ESR1 mutation detection in liquid biopsy of metastatic breast cancer patients
- PMID: 39392509
- PMCID: PMC12546375
- DOI: 10.1007/s00428-024-03942-1
Diagnostic utility of ESR1 mutation detection in liquid biopsy of metastatic breast cancer patients
Abstract
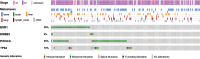
Molecular analysis of circulating cell-free DNA (cfDNA) extracted from peripheral blood plasma samples of metastatic breast cancer (BC) patients is of rising interest to find optimal therapeutic strategies. Detection of emerging resistance mutations against endocrine therapy is possible with this approach. Here we present the applicability of a laboratory-developed NGS assay in molecular pathology routine diagnostic, covering four genes with therapeutic (ESR1, PIK3CA, ERBB2) and prognostic (TP53) consequences in metastatic BC. We analyzed 162 liquid biopsy samples and 25 corresponding metastases from metastatic BC patients. In the liquid biopsies, we detected ESR1 mutations in 42 cases (25.9%) and ERBB2 mutations in six cases (3.7%), arguing for a change in therapy to fulvestrant, elacestrant, or neratinib. Furthermore, 17 cases had detectable TP53 mutations, associated with resistance against endocrine therapy. We conclude that liquid biopsy testing is a noninvasive, sensitive, and helpful method to optimize therapeutic decisions in metastatic BC.
Keywords: ESR1; Breast cancer; Elacestrant; Liquid biopsy; Metastasis.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: Retrospective analyses of anonymized diagnostic left over material have been approved by the local ethics committee (Hannover Medical School, Germany, Head: Prof. Schmidt). Conflict of interest: The authors declare no competing interests.
Figures


References
-
- Bidard FC, Hardy-Bessard AC, Dalenc F et al (2022) Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 11:1367–1377. 10.1016/S1470-2045(22)00555-1 - DOI - PubMed
-
- Bidard FC, Kaklamani VG, Neven P et al (2022) Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: results from the randomized phase III EMERALD trial. JCO 28:3246–3256. 10.1200/JCO.22.00338 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

