Electronic Nudges to Increase Influenza Vaccination in Patients With Chronic Diseases: A Randomized Clinical Trial
- PMID: 39392741
- PMCID: PMC11581639
- DOI: 10.1001/jama.2024.21060
Electronic Nudges to Increase Influenza Vaccination in Patients With Chronic Diseases: A Randomized Clinical Trial
Abstract
Importance: Despite strong worldwide guideline recommendations, influenza vaccination rates remain suboptimal among young and middle-aged patients with chronic diseases. Effective scalable strategies to increase vaccination are needed.
Objective: To investigate whether electronically delivered letter-based nudges informed by behavioral science could increase influenza vaccination uptake among patients aged 18 to 64 years with chronic diseases.
Design, setting, and participants: Nationwide pragmatic registry-based randomized clinical implementation trial conducted between September 24, 2023, and May 31, 2024, enrolling all Danish citizens aged 18 to 64 years who met criteria for free-of-charge influenza vaccination in light of preexisting chronic disease. All trial data were sourced from nationwide administrative health registries.
Intervention: Randomized in 2.45:1:1:1:1:1:1 ratio to no letter (usual care) or 6 different behaviorally informed electronic letters.
Main outcomes and measures: The primary end point was receipt of influenza vaccination on or before January 1, 2024, assessed in 7 prespecified coprimary comparisons (all intervention groups pooled vs usual care and each individual intervention group vs usual care). Absolute risk difference in proportions and a crude relative risk were calculated for each comparison.
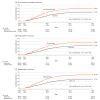
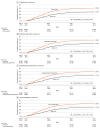
Results: A total of 299 881 participants (53.2% [159 454] female, median age, 52.0 [IQR, 39.8-59.0] years) were randomized. Compared with usual care, influenza vaccination rates were higher among those receiving any intervention letter (any intervention letter, 39.6% vs usual care, 27.9%; difference, 11.7 percentage points; 99.29% CI, 11.2-12.2 percentage points; P < .001). Each individual letter type significantly increased influenza vaccination with the largest effect sizes observed with a repeated letter sent 10 days after the initial letter (repeated letter, 41.8% vs usual care, 27.9%; difference, 13.9 percentage points; 99.29% CI, 13.1-14.7 percentage points; P < .001) and a letter emphasizing potential cardiovascular benefits of vaccination (cardiovascular gain, 39.8% vs usual care, 27.9%; difference, 11.9 percentage points; 99.29% CI, 11.1-12.7 percentage points; P < .001). Vaccination rates were improved across major subgroups.
Conclusions and relevance: In a nationwide randomized clinical implementation trial, electronically delivered letter-based nudges markedly increased influenza vaccination compared with usual care among young and middle-aged patients with chronic diseases. The results of this study suggest that simple, scalable, and cost-efficient electronic letter strategies may have substantial public health implications.
Trial registration: ClinicalTrials.gov Identifier: NCT06030739.
Conflict of interest statement
Figures



References
-
- World Health Organization. Influenza (seasonal). October 3, 2023. Accessed January 19, 2024. https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
-
- Rondy M, El Omeiri N, Thompson MG, Levêque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect. 2017;75(5):381-394. doi: 10.1016/j.jinf.2017.09.010 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

