IFN-γ primes bone marrow neutrophils to acquire regulatory functions in severe viral respiratory infections
- PMID: 39392875
- PMCID: PMC11468905
- DOI: 10.1126/sciadv.adn3257
IFN-γ primes bone marrow neutrophils to acquire regulatory functions in severe viral respiratory infections
Abstract
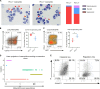
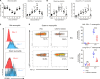
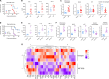
Neutrophil subsets endowed with regulatory/suppressive properties are widely regarded as deleterious immune cells that can jeopardize antitumoral response and/or antimicrobial resistance. Here, we describe a sizeable fraction of neutrophils characterized by the expression of programmed death-ligand 1 (PD-L1) in biological fluids of humans and mice with severe viral respiratory infections (VRI). Biological and transcriptomic approaches indicated that VRI-driven PD-L1+ neutrophils are endowed with potent regulatory functions and reduced classical antimicrobial properties, as compared to their PD-L1- counterpart. VRI-induced regulatory PD-L1+ neutrophils were generated remotely in the bone marrow in an IFN-γ-dependent manner and were quickly mobilized into the inflamed lungs where they fulfilled their maturation. Neutrophil depletion and PD-L1 blockade during experimental VRI resulted in higher mortality, increased local inflammation, and reduced expression of resolving factors. These findings suggest that PD-L1+ neutrophils are important players in disease tolerance by mitigating local inflammation during severe VRI and that they may constitute relevant targets for future immune interventions.
Figures





References
-
- Thompson B. T., Chambers R. C., Liu K. D., Acute respiratory distress syndrome. N. Engl. J. Med. 377, 562–572 (2017). - PubMed
-
- Ahuja S. K., Manoharan M. S., Lee G. C., McKinnon L. R., Meunier J. A., Steri M., Harper N., Fiorillo E., Smith A. M., Restrepo M. I., Branum A. P., Bottomley M. J., Orrù V., Jimenez F., Carrillo A., Pandranki L., Winter C. A., Winter L. A., Gaitan A. A., Moreira A. G., Walter E. A., Silvestri G., King C. L., Zheng Y.-T., Zheng H.-Y., Kimani J., Blake Ball T., Plummer F. A., Fowke K. R., Harden P. N., Wood K. J., Ferris M. T., Lund J. M., Heise M. T., Garrett N., Canady K. R., Abdool Karim S. S., Little S. J., Gianella S., Smith D. M., Letendre S., Richman D. D., Cucca F., Trinh H., Sanchez-Reilly S., Hecht J. M., Cadena Zuluaga J. A., Anzueto A., Pugh J. A., South Texas Veterans Health Care System COVID-19 team, Abdalla M. I., Adams S. G., Adebayo Y., Agnew J., Ali S., Anstead G., Balmes M., Barker J., Baruch-Bienen D., Bible V., Birdwell A., Braddy S., Bradford S., Briggs H., Corral J. M., Dacus J. J., Danaher P. J., DePaul S. A., Dickerson J., Doanne J., Ehsan A., Elbel S., Escalante M., Escamilla C., Escamilla V., Farrar R., Feldman D., Flores D., Flynn J., Ford D., Foy J. D., Freeman M., Galley S., Garcia J., Garza M., Gilman S., Goel M., Gomez J., Goyal V. K., Grassmuck S., Grigsby S., Hanson J., Harris B., Haywood A., Hinojosa C., Ho T. T., Hopkins T., Horvath L. L., Hussain A. N., Jabur A., Jewell P., Johnson T. B., Lawler A. C., Lee M., Lester C. S., Levine S. M., Lewis H. V., Louder A., Mainor C., Maldonado R., Martinez C., Martinez Y., Maselli D., Mata C., McElligott N., Medlin L., Mireles M., Moreno J., Morneau K., Muetz J., Munro S. B., Murray C., Nambiar A., Nassery D., Nathanson R., Oakman K., O’Rorke J., Padgett C., Pascual-Guardia S., Patterson M., Perez G. L., Perez R., Perez R. III, Phillips R. E., Polk P. B., Pomager M. A., Preston K. J., Proud K. C., Rangel M., Ratcliffe T. A., Reichelderfer R. L., Renz E. M., Ross J., Rudd T., Sanchez M. E., Sanders T., Schindler K. C., Schmit D., Sehgal R. T., Solorzano C., Soni N., Tam W. S., Tovar E. J., Trammell Velasquez S. A., Tyler A. R., Vasquez A., Veloso M. C., Venticinque S. G., Villalpando J. A., Villanueva M., Villegas L., Walker M., Wallace A., Wallace M., Wang E., Wickizer S., Williamson A., Yunes A., Zentner K. H., Agan B. K., Root-Bernstein R., Clark R. A., Okulicz J. F., He W., Immune resilience despite inflammatory stress promotes longevity and favorable health outcomes including resistance to infection. Nat Commun 14, 3286 (2023). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

