Shank3 mutation impairs glutamate signaling and myelination in ASD mouse model and human iPSC-derived OPCs
- PMID: 39392881
- PMCID: PMC11468907
- DOI: 10.1126/sciadv.adl4573
Shank3 mutation impairs glutamate signaling and myelination in ASD mouse model and human iPSC-derived OPCs
Abstract
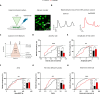
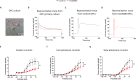
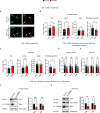
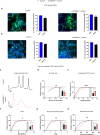
Autism spectrum disorder (ASD) is characterized by social and neurocognitive impairments, with mutations of the SHANK3 gene being prominent in patients with monogenic ASD. Using the InsG3680 mouse model with a Shank3 mutation seen in humans, we revealed an unknown role for Shank3 in postsynaptic oligodendrocyte (OL) features, similar to its role in neurons. This was shown by impaired molecular and physiological glutamatergic traits of InsG3680-derived primary OL cultures. In vivo, InsG3680 mice exhibit significant reductions in the expression of key myelination-related transcripts and proteins, along with deficits in myelin ultrastructure, white matter, axonal conductivity, and motor skills. Last, we observed significant impairments, with clinical relevance, in induced pluripotent stem cell-derived OLs from a patient with the InsG3680 mutation. Together, our study provides insight into Shank3's role in OLs and reveals a mechanism of the crucial connection of myelination to ASD pathology.
Figures





References
-
- Amaral D. G., Schumann C. M., Nordahl C. W., Neuroanatomy of autism. Trends Neurosci. 31, 137–145 (2008). - PubMed
-
- Lord C., Cook E. H., Leventhal B. L., Amaral D. G., Autism spectrum disorders. Neuron 28, 355–363 (2000). - PubMed
-
- Huguet G., Ey E., Bourgeron T., The genetic landscapes of autism spectrum disorders. Annu. Rev. Genom. Hum. Genet. 14, 191–213 (2013). - PubMed
-
- Satterstrom F. K., Kosmicki J. A., Wang J., Breen M. S., De Rubeis S., An J. Y., Peng M., Collins R., Grove J., Klei L., Stevens C., Reichert J., Mulhern M. S., Artomov M., Gerges S., Sheppard B., Xu X., Bhaduri A., Norman U., Brand H., Schwartz G., Nguyen R., Guerrero E. E., Dias C., Betancur C., Cook E. H., Gallagher L., Gill M., Sutcliffe J. S., Thurm A., Zwick M. E., Børglum A. D., State M. W., Cicek A. E., Talkowski M. E., Cutler D. J., Devlin B., Sanders S. J., Roeder K., Daly M. J., Buxbaum J. D., Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell 180, 568–584.e23 (2020). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

