Mechanism of degrader-targeted protein ubiquitinability
- PMID: 39392888
- PMCID: PMC11468923
- DOI: 10.1126/sciadv.ado6492
Mechanism of degrader-targeted protein ubiquitinability
Abstract
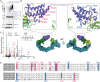
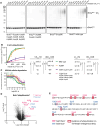
Small-molecule degraders of disease-driving proteins offer a clinically proven modality with enhanced therapeutic efficacy and potential to tackle previously undrugged targets. Stable and long-lived degrader-mediated ternary complexes drive fast and profound target degradation; however, the mechanisms by which they affect target ubiquitination remain elusive. Here, we show cryo-EM structures of the VHL Cullin 2 RING E3 ligase with the degrader MZ1 directing target protein Brd4BD2 toward UBE2R1-ubiquitin, and Lys456 at optimal positioning for nucleophilic attack. In vitro ubiquitination and mass spectrometry illuminate a patch of favorably ubiquitinable lysines on one face of Brd4BD2, with cellular degradation and ubiquitinomics confirming the importance of Lys456 and nearby Lys368/Lys445, identifying the "ubiquitination zone." Our results demonstrate the proficiency of MZ1 in positioning the substrate for catalysis, the favorability of Brd4BD2 for ubiquitination by UBE2R1, and the flexibility of CRL2 for capturing suboptimal lysines. We propose a model for ubiquitinability of degrader-recruited targets, providing a mechanistic blueprint for further rational drug design.
Figures




References
-
- Zheng N., Shabek N., Ubiquitin ligases: Structure, function, and regulation. Annu. Rev. Biochem. 86, 129–157 (2017). - PubMed
-
- Cowan A. D., Ciulli A., Driving E3 ligase substrate specificity for targeted protein degradation: Lessons from nature and the laboratory. Annu. Rev. Biochem. 91, 295–319 (2022). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

