Synergistic combination of perphenazine and temozolomide suppresses patient-derived glioblastoma tumorspheres
- PMID: 39392921
- PMCID: PMC11889716
- DOI: 10.1093/neuonc/noae211
Synergistic combination of perphenazine and temozolomide suppresses patient-derived glioblastoma tumorspheres
Abstract
Background: Glioblastoma (GBM), a primary malignant brain tumor, has a poor prognosis, even with standard treatments such as radiotherapy and chemotherapy. In this study, we explored the anticancer effects of the synergistic combination of perphenazine (PER), a dopamine receptor D2/3 (DRD2/3) antagonist, and temozolomide (TMZ), a standard treatment for GBM, in patient-derived human GBM tumorspheres (TSs).
Methods: The biological effects of the combination of PER and TMZ in GBM TSs were assessed by measuring cell viability, ATP, stemness, invasiveness, and apoptosis. Changes in protein and mRNA expression were analyzed using western blotting and RNA sequencing. Co-administration of PER and TMZ was evaluated in vivo using a mouse orthotopic xenograft model.
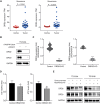
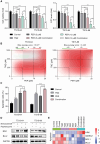
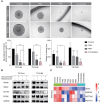
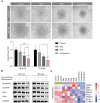
Results: The Severance dataset showed that DRD2 and DRD3 expressions were higher in tumor tissues than in the tumor-free cortex of patients with GBM. DRD2/3 knockout by CRISPR/Cas9 in patient-derived human GBM TSs inhibited cell growth and ATP production. The combined treatment with PER and TMZ resulted in superior effects on cell viability and ATP assays compared to those in single treatment groups. Flow cytometry, western blotting, and RNA sequencing confirmed elevated apoptosis in GBM TSs following combination treatment. Additionally, the combination of PER and TMZ downregulated the expression of protein and mRNA associated with stemness and invasiveness. In vivo evaluation showed that combining PER and TMZ extended the survival period of the mouse orthotopic xenograft model.
Conclusions: The synergistic combination of PER and TMZ has potential as a novel combination treatment strategy for GBM.
Keywords: DRD2/3; glioblastoma; perphenazine; temozolomide; tumorsphere.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
None declared.
Figures
References
-
- Hoshide R, Jandial R.. 2016 World Health Organization classification of central nervous system tumors: an era of molecular biology. World Neurosurg. 2016;94:561–562. - PubMed
-
- Stupp R, Hegi ME, Mason WP, et al.; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. - PubMed
-
- Roh TH, Kang SG, Moon JH, et al.Survival benefit of lobectomy over gross-total resection without lobectomy in cases of glioblastoma in the noneloquent area: a retrospective study. J Neurosurg. 2019;132(3):895–901. - PubMed
-
- Bertolini F, Sukhatme VP, Bouche G.. Drug repurposing in oncology--patient and health systems opportunities. Nat Rev Clin Oncol. 2015;12(12):732–742. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

