Adjuvant Nivolumab in High-Risk Muscle-Invasive Urothelial Carcinoma: Expanded Efficacy From CheckMate 274
- PMID: 39393026
- PMCID: PMC11687940
- DOI: 10.1200/JCO.24.00340
Adjuvant Nivolumab in High-Risk Muscle-Invasive Urothelial Carcinoma: Expanded Efficacy From CheckMate 274
Abstract
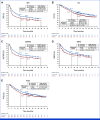
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.CheckMate 274 is a phase III, randomized, double-blind trial of adjuvant nivolumab versus placebo for muscle-invasive urothelial carcinoma (MIUC) at high risk of recurrence after radical resection. The primary end points of disease-free survival (DFS) in intent-to-treat (ITT) and tumor PD-L1 expression ≥1% populations were met. We report results at an extended median follow-up of 36.1 months in the ITT population. In addition, we report interim overall survival (OS) data for the first time and an exploratory analysis among patients with bladder primary tumors (muscle-invasive bladder cancer [MIBC]). Consistent DFS benefit with nivolumab versus placebo was observed in both the ITT (hazard ratio [HR], 0.71 [95% CI, 0.58 to 0.86]) and PD-L1 ≥1% (HR, 0.52 [95% CI, 0.37 to 0.72]) patients. The HR for OS with nivolumab versus placebo was 0.76 (95% CI, 0.61 to 0.96) in the ITT population and 0.56 (95% CI, 0.36 to 0.86) in the PD-L1 ≥1 population. Continuous benefit in nonurothelial tract recurrence-free survival and distant metastasis-free survival was also observed in both patient populations. The exploratory analysis of patients with MIBC also showed continued efficacy benefits, irrespective of PD-L1 status. No new safety signals were reported. Overall, these results further support adjuvant nivolumab as a standard of care for high-risk MIUC after radical resection.
Trial registration: ClinicalTrials.gov NCT02632409.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- Kim HS, Jeong CW, Kwak C, et al. Disease-free survival at 2 and 3 years is a significant early surrogate marker predicting the 5-year overall survival in patients treated with radical cystectomy for urothelial carcinoma of the bladder: External evaluation and validation in a cohort of Korean patients. Front Oncol. 2015;5:246. - PMC - PubMed
-
- Bellmunt J, Valderrama BP, Puente J, et al. Recent therapeutic advances in urothelial carcinoma: A paradigm shift in disease management. Crit Rev Oncol Hematol. 2022;174:103683. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

