Pharmacokinetics and Pharmacodynamics of Natalizumab 6-Week Dosing vs Continued 4-Week Dosing for Relapsing-Remitting Multiple Sclerosis
- PMID: 39393045
- PMCID: PMC11488827
- DOI: 10.1212/NXI.0000000000200321
Pharmacokinetics and Pharmacodynamics of Natalizumab 6-Week Dosing vs Continued 4-Week Dosing for Relapsing-Remitting Multiple Sclerosis
Erratum in
-
Pharmacokinetics and Pharmacodynamics of Natalizumab 6-Week Dosing vs Continued 4-Week Dosing for Relapsing-Remitting Multiple Sclerosis.Neurol Neuroimmunol Neuroinflamm. 2025 Jan;12(1):e200354. doi: 10.1212/NXI.0000000000200354. Epub 2024 Dec 4. Neurol Neuroimmunol Neuroinflamm. 2025. PMID: 39631033 Free PMC article. No abstract available.
Abstract
Background and objectives: Exposure to natalizumab, an efficacious treatment for relapsing-remitting multiple sclerosis (RRMS), is associated with increased risk of progressive multifocal leukoencephalopathy (PML). Compared with every-4-week (Q4W) dosing, extended-interval dosing of natalizumab is associated with decreased risk of PML. Clinical efficacy was maintained in the majority of patients switched to every-6-week (Q6W) dosing in the phase 3b NOVA clinical trial. In this article, we report pharmacokinetics (PK) and pharmacodynamics (PD) of Q6W vs Q4W dosing in NOVA.
Methods: In NOVA study Part 1, participants with RRMS (aged 18-60 years) and Expanded Disability Status Scale score <5.5, who were stable on IV natalizumab Q4W dosing for ≥12 months, were randomized to continue IV Q4W dosing or switched to IV Q6W dosing of natalizumab and followed for 72 weeks. Exploratory outcomes were measurements of trough serum natalizumab concentration, α4-integrin saturation, and soluble vascular cell adhesion molecule-1 (sVCAM-1) concentration. A mixed model of repeated measures was used to estimate mean treatment differences between groups. Patient-level PK and PD data were examined in those with relapse or radiologic disease activity.
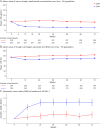
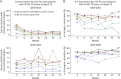
Results: In NOVA, 486 (Q6W, n = 245; Q4W, n = 241) and 487 (Q6W, n = 246; Q4W, n = 241) participants were included in the PK and PD populations, respectively. Mean trough natalizumab concentrations ranged from 10 to 21 μg/mL (Q6W) and 33-38 μg/mL (Q4W), and mean α4-integrin saturation remained above 65.5% (Q6W) and above 77.9% (Q4W). In the Q6W group, mean sVCAM-1 levels increased 23.6% by week 24 and remained elevated throughout the study, while mean sVCAM-1 levels remained generally stable in the Q4W group. Most participants with T2 lesion activity or relapse activity, in either treatment arm, maintained trough natalizumab levels >10 μg/mL and trough α4-integrin saturation >50%.
Discussion: Compared with Q4W dosing, Q6W dosing was associated with a 60%-70% decrease in mean trough natalizumab levels and a 9%-16% decrease in mean α4-integrin saturation. At the patient level, neither natalizumab concentration nor α4-integrin saturation was consistently predictive of lesion or relapse activity, suggesting that trough natalizumab and α4-integrin saturation measurements should be interpreted with caution in clinical practice.
Trial registration information: ClinicalTrials.gov, NCT03689972; EudraCT, 2018-002145-11. Submitted 2018-09-27. First patient enrolled: 2018-12-26. https://clinicaltrials.gov/study/NCT03689972.
Conflict of interest statement
J.F. Foley reports personal compensation for consulting activities from Biogen, Octave and compensation (paid to institution) for data safety monitoring or advisory boards from Biogen, Genentech, Novartis; G. Defer reports personal compensation for scientific advisory boards and funding for travel and/or speaker honoraria from Biogen, Bristol Myers Squibb, Merck Serono, Novartis, Sanofi Genzyme, Teva Pharmaceuticals and research grants (paid to institution) from Biogen, Merck Serono, Novartis, Sanofi Genzyme; L.Z. Ryerson reports personal compensation for advisory board activities from Biogen, Genentech, Novartis and research support from Biogen, Celgene, Genentech; J.A. Cohen reports personal compensation for consulting from Astoria, Bristol Myers Squibb, Convelo, EMD Serono, FiND Therapeutics, INMune, Sandoz; and serves as an Editor of
Figures

References
-
- Biogen Inc. Tysabri (natalizumab) [prescribing information]. Accessed March 31, 2023. tysabri.com/content/dam/commercial/tysabri/pat/en_us/pdf/tysabri_prescri...
-
- Bochner BS, Luscinskas FW, Gimbrone MA Jr, et al. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med. 1991;173(6):1553-1557. doi: 10.1084/jem.173.6.1553 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
