Pediatric MOG-Ab-Associated Encephalitis: Supporting Early Recognition and Treatment
- PMID: 39393046
- PMCID: PMC11488826
- DOI: 10.1212/NXI.0000000000200323
Pediatric MOG-Ab-Associated Encephalitis: Supporting Early Recognition and Treatment
Erratum in
-
Pediatric MOG-Ab-Associated Encephalitis: Supporting Early Recognition and Treatment.Neurol Neuroimmunol Neuroinflamm. 2025 Jan;12(1):e200348. doi: 10.1212/NXI.0000000000200348. Epub 2024 Nov 18. Neurol Neuroimmunol Neuroinflamm. 2025. PMID: 39556783 Free PMC article. No abstract available.
Abstract
Background and objectives: Antibodies to myelin oligodendrocyte glycoprotein (MOG-Ab) have recently been reported in patients with encephalitis who do not fulfill criteria for acute disseminated encephalomyelitis (ADEM). We evaluated a cohort of these children and compared them with children with ADEM.
Methods: This retrospective, multicenter cohort study comprised consecutive patients <18 years of age with MOG-Ab who fulfilled criteria for autoimmune encephalitis. These patients were stratified into (1) children not fulfilling criteria for ADEM (encephalitis phenotype) and (2) children with ADEM. Clinical/paraclinical data were extracted from the electronic records. Comparisons were made using the Mann-Whitney U test and χ2 Fisher exact test for statistical analysis.
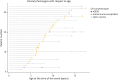
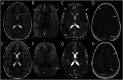
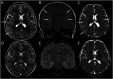
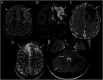
Results: From 235 patients with positive MOG-Ab, we identified 33 (14%) with encephalitis and 74 (31%) with ADEM. The most common presenting symptoms in children with encephalitis were headache (88%), seizures (73%), and fever (67%). Infective meningoencephalitis was the initial diagnosis in 67%. CSF pleocytosis was seen in 79%. Initial MRI brain was normal in 8/33 (24%) patients. When abnormal, multifocal cortical changes were seen in 66% and unilateral cortical changes in 18%. Restricted diffusion was demonstrated in 43%. Intra-attack new lesions were seen in 7/13 (54%). When comparing with children with ADEM, children with encephalitis were older (median 8.9 vs 5.7 years, p = 0.005), were more likely to be admitted to intensive care (14/34 vs 4/74, p < 0.0001), were given steroid later (median 16.6 vs 9.6 days, p = 0.04), and were more likely to be diagnosed with epilepsy at last follow-up (6/33 vs 1/74, p = 0.003).
Discussion: MOG-Ab should be tested in all patients with suspected encephalitis even in the context of initially normal brain MRI. Although exclusion of infections should be part of the diagnostic process of any child with encephalitis, in immunocompetent children, when herpes simplex virus CSF PCR and gram stains are negative, these features do not preclude the diagnosis of immune mediated disease and should not delay initiation of first-line immunosuppression (steroids, IVIG, plasma exchange), even while awaiting the antibody results.
Conflict of interest statement
O. Abdel-Mannan receives funding from the Association British Neurologists, MS Society and the Berkeley Foundation’s AW Pidgley Memorial Trust. O. Cicarelli is NIHR Research Professor (RP - 2017-08-ST2-004). She also receives funding from MRC, United Kingdom and National MS Society and, NIHR and Rosetrees Trust. She is a member of independent DSMB for Novartis, gave a teaching talk on McDonald criteria in a Merck local symposium, and contributed to an Advisory Board for Biogen; she is Deputy Editor of
Figures

References
-
- Hacohen Y, Wright S, Waters P, et al. Paediatric autoimmune encephalopathies: clinical features, laboratory investigations and outcomes in patients with or without antibodies to known central nervous system autoantigens. J Neurol Neurosurg Psychiatry. 2013;84(7):748-755. doi: 10.1136/jnnp-2012-303807. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
