A Texting- and Internet-Based Self-Reporting System for Enhanced Vaccine Safety Surveillance With Insights From a Large Integrated Health Care System in the United States: Prospective Cohort Study
- PMID: 39393058
- PMCID: PMC11512128
- DOI: 10.2196/58991
A Texting- and Internet-Based Self-Reporting System for Enhanced Vaccine Safety Surveillance With Insights From a Large Integrated Health Care System in the United States: Prospective Cohort Study
Abstract
Background: SMS text messaging- and internet-based self-reporting systems can supplement existing vaccine safety surveillance systems, but real-world participation patterns have not been assessed at scale.
Objective: This study aimed to describe the participation rates of a new SMS text messaging- and internet-based self-reporting system called the Kaiser Permanente Side Effect Monitor (KPSEM) within a large integrated health care system.
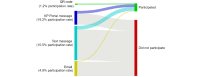
Methods: We conducted a prospective cohort study of Kaiser Permanente Southern California (KPSC) patients receiving a COVID-19 vaccination from April 23, 2021, to July 31, 2023. Patients received invitations through flyers, SMS text messages, emails, or patient health care portals. After consenting, patients received regular surveys to assess adverse events up to 5 weeks after each dose. Linkage with medical records provided demographic and clinical data. In this study, we describe KPSEM participation rates, defined as providing consent and completing at least 1 survey within 35 days of COVID-19 vaccination.
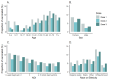
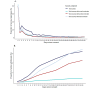
Results: Approximately, 8% (164,636/2,091,975) of all vaccinated patients provided consent and completed at least 1 survey within 35 days. The lowest participation rates were observed for parents of children aged 12-17 years (1349/152,928, 0.9% participation rate), and the highest participation was observed among older adults aged 61-70 years (39,844/329,487, 12.1%). Persons of non-Hispanic White race were more likely to participate compared with other races and ethnicities (13.1% vs 3.9%-7.5%, respectively; P<.001). In addition, patients residing in areas with a higher neighborhood deprivation index were less likely to participate (5.1%, 16,503/323,122 vs 10.8%, 38,084/352,939 in the highest vs lowest deprivation quintiles, respectively; P<.001). Invitations through the individual's Kaiser Permanente health care portal account and by SMS text message were associated with the highest participation rate (19.2%, 70,248/366,377 and 10.5%, 96,169/914,793, respectively), followed by email (19,464/396,912, 4.9%) and then QR codes on flyers (25,882/2,091,975, 1.2%). SMS text messaging-based surveys demonstrated the highest sustained daily response rates compared with internet-based surveys.
Conclusions: This real-world prospective study demonstrated that a novel digital vaccine safety self-reporting system implemented through an integrated health care system can achieve high participation rates. Linkage with participants' electronic health records is another unique benefit of this surveillance system. We also identified lower participation among selected vulnerable populations, which may have implications when interpreting data collected from similar digital systems.
Keywords: COVID-19 vaccination; COVID-19 vaccines; EHR; US; USA; cohort study; data collection; digital health; disparity; electronic health records; internet based; medical records; mobile phone; monitoring; self-reporting; surveillance; surveillance system; survey; survey participation; surveys; text; text message; text-based surveys; vaccination; vaccine; vaccine monitoring; vaccine safety; vaccine safety monitoring; vulnerable.
©Debbie E Malden, Julianne Gee, Sungching Glenn, Zhuoxin Li, Denison S Ryan, Zheng Gu, Cassandra Bezi, Sunhea Kim, Amelia Jazwa, Michael M McNeil, Eric S Weintraub, Sara Y Tartof. Originally published in JMIR mHealth and uHealth (https://mhealth.jmir.org), 11.10.2024.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- Pew Research Center Mobile fact sheet. [2024-01-31]. https://www.pewresearch.org/internet/fact-sheet/mobile/
-
- Zhou W, Pool V, Iskander JK, English-Bullard R, Ball R, Wise RP, Haber P, Pless RP, Mootrey G, Ellenberg SS, Braun MM, Chen RT. Surveillance for safety after immunization: vaccine adverse event reporting system (VAERS)--United States, 1991-2001. MMWR Surveill Summ. 2003;52(1):1–24. http://www.cdc.gov/mmwr/preview/mmwrhtml/ss5201a1.htm - PubMed
-
- Bota AB, Bettinger JA, Sarfo-Mensah S, Lopez J, Smith DP, Atkinson KM, Bell C, Marty K, Serhan M, Zhu DT, McCarthy AE, Wilson K. Comparing the use of a mobile app and a web-based notification platform for surveillance of adverse events following influenza immunization: randomized controlled trial. JMIR Public Health Surveill. 2023;9:e39700. doi: 10.2196/39700. https://publichealth.jmir.org/2023//e39700/ v9i1e39700 - DOI - PMC - PubMed
-
- Leigh JW, Gerber BS, Gans CP, Kansal MM, Kitsiou S. Smartphone ownership and interest in mobile health technologies for self-care among patients with chronic heart failure: cross-sectional survey study. JMIR Cardio. 2022;6(1):e31982. doi: 10.2196/31982. https://cardio.jmir.org/2022/1/e31982/ v6i1e31982 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

