A quantitative proteomic approach to evaluate the efficacy of carnosine in a murine model of chronic obstructive pulmonary disease (COPD)
- PMID: 39393288
- PMCID: PMC11663752
- DOI: 10.1016/j.redox.2024.103374
A quantitative proteomic approach to evaluate the efficacy of carnosine in a murine model of chronic obstructive pulmonary disease (COPD)
Abstract
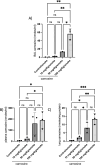
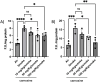
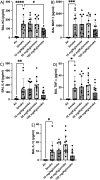
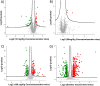
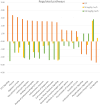
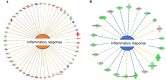
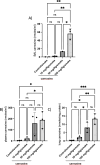
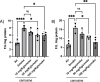
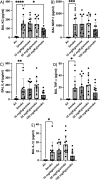
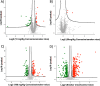
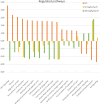
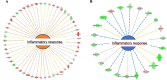
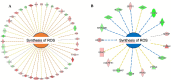
The aim of the work was to study a dose-dependent effect of inhaled carnosine (10, 50 or 100 mg/kg/day) in mice exposed to cigarette smoke as a model of chronic obstructive pulmonary disease (COPD). A dose-dependent loading of the dipeptide in lung tissue and bronchoalveolar lavage (BAL) was firstly demonstrated by LC-ESI-MS analysis. Cigarette smoke exposure induced a significant lung inflammation and oxidative stress in mice which was dose-dependently reduced by carnosine. Inflammation was firstly evaluated by measuring the cytokines content in the BAL. All the measured cytokines were found significantly higher in the smoke group in respect to control, although the data are affected by a significant variability. Carnosine was found effective only at the highest dose tested and significantly only for keratinocyte-derived cytokine (KC). Due to the high variability of cytokines, a quantitative proteomic approach to better understand the functional effect of carnosine and its molecular mechanisms was used. Proteomic data clearly indicate that smoke exposure had a great impact on lung tissue with 692 proteins differentially expressed above a threshold of 1.5-fold. Protein network analysis identified the activation of some pathways characteristic of COPD, including inflammatory response, fibrosis, induction of immune system by infiltration and migration of leukocyte pathways, altered pathway of calcium metabolism and oxidative stress. Carnosine at the tested dose of 100 mg/kg was found effective in reverting all the pathways evoked by smoke. Only a partial reverse of the dysregulated proteins was evident at low- and mid-tested doses, although, for some specific proteins, indicating an overall dose-dependent effect. Regarding the molecular mechanisms involved, we found that carnosine upregulated some key enzymes related to Nrf2 activation and in particular glutathione peroxidase, reductase, transferase, SOD, thioredoxins, and carbonyl reductase. Such mechanism would explain the antioxidant and anti-inflammatory effects of the dipeptide.
Keywords: Carnosine; Chronic obstructive pulmonary disease (COPD); Cigarette smoke; Inflammation; Nrf2; Oxidative stress; Quantitative proteomic.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The following Authors, Elsa Melloni, Gloria Padoani, Silvia Vailati, Giovanni Caponetti are employees of Zambon SpA. Giancarlo Aldini is PI of a research contract signed by the Università degli Studi di Milano with Zambon S.p.A (CTE_NAZPR20GALDI_04) which partially supported the study. Zambon S.p.a. is the owner of the airway administration technology, Edry™, which was applied in the present study to deliver carnosine to the lung.
Figures


References
-
- Christenson S.A., Smith B.M., Bafadhel M., Putcha N. Chronic obstructive pulmonary disease. Lancet. 2022;399(10342):2227–2242. - PubMed
-
- Hogea S.P., Tudorache E., Fildan A.P., Fira-Mladinescu O., Marc M., Oancea C. Risk factors of chronic obstructive pulmonary disease exacerbations. Clin. Res. J. 2020;14(3):183–197. - PubMed
-
- Li S., Huang Q., He B. SIRT1 as a potential therapeutic target for chronic obstructive pulmonary disease. Lung. 2023;201(2):201–215. - PubMed

