Accelerated mitochondrial dynamics promote spermatogonial differentiation
- PMID: 39393359
- PMCID: PMC11589200
- DOI: 10.1016/j.stemcr.2024.09.006
Accelerated mitochondrial dynamics promote spermatogonial differentiation
Abstract
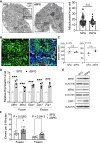
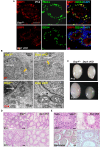
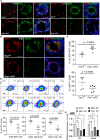
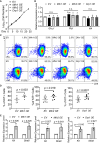
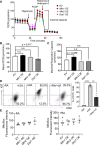
At different stages of spermatogenesis, germ cell mitochondria differ remarkably in morphology, architecture, and functions. However, it remains elusive how mitochondria change their features during spermatogonial differentiation, which in turn impacts spermatogonial stem cell fate decision. In this study, we observed that mitochondrial fusion and fission were both upregulated during spermatogonial differentiation. As a result, the mitochondrial morphology remained unaltered. Enhanced mitochondrial fusion and fission promoted spermatogonial differentiation, while the deficiency in DRP1-mediated fission led to a stage-specific blockage of spermatogenesis at differentiating spermatogonia. Our data further revealed that increased expression of pro-fusion factor MFN1 upregulated mitochondrial metabolism, whereas DRP1 specifically regulated mitochondrial permeability transition pore opening in differentiating spermatogonia. Taken together, our findings unveil how proper spermatogonial differentiation is precisely controlled by concurrently accelerated and properly balanced mitochondrial fusion and fission in a germ cell stage-specific manner, thereby providing critical insights about mitochondrial contribution to stem cell fate decision.
Keywords: DRP1; MFN1; mitochondrial dynamics; spermatogonial differentiation; spermatogonial stem cells.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
-
- Alexander C., Votruba M., Pesch U.E., Thiselton D.L., Mayer S., Moore A., Rodriguez M., Kellner U., Leo-Kottler B., Auburger G., et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat. Genet. 2000;26:211–215. - PubMed
-
- Buaas F.W., Kirsh A.L., Sharma M., McLean D.J., Morris J.L., Griswold M.D., De Rooij D.G., Braun R.E. Plzf is required in adult male germ cells for stem cell self-renewal. Nat. Genet. 2004;36:647–652. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

