A nonnatural peptide targeting the A-kinase anchoring function of PI3Kγ for therapeutic cAMP modulation in pulmonary cells
- PMID: 39393573
- PMCID: PMC11585760
- DOI: 10.1016/j.jbc.2024.107873
A nonnatural peptide targeting the A-kinase anchoring function of PI3Kγ for therapeutic cAMP modulation in pulmonary cells
Abstract
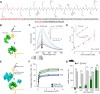
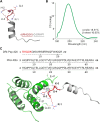
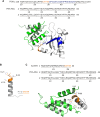
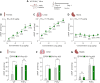
A-kinase anchoring proteins (AKAPs) are key orchestrators of cAMP signaling that act by recruiting protein kinase A (PKA) in proximity of its substrates and regulators to specific subcellular compartments. Modulation of AKAPs function offers the opportunity to achieve compartment-restricted modulation of the cAMP/PKA axis, paving the way to new targeted treatments. For instance, blocking the AKAP activity of phosphoinositide 3-kinase γ (PI3Kγ) improves lung function by inducing cAMP-mediated bronchorelaxation, ion transport, and antiinflammatory responses. Here, we report the generation of a nonnatural peptide, D-retroinverso (DRI)-Pep #20, optimized to disrupt the AKAP function of PI3Kγ. DRI-Pep #20 mimicked the native interaction between the N-terminal domain of PI3Kγ and PKA, demonstrating nanomolar affinity for PKA, high resistance to protease degradation and high permeability to the pulmonary mucus barrier. DRI-Pep #20 triggered cAMP elevation both in vivo in the airway tract of mice upon intratracheal administration, and in vitro in bronchial epithelial cells of cystic fibrosis (CF) patients. In CF cells, DRI-Pep #20 rescued the defective function of the cAMP-operated channel cystic fibrosis transmembrane conductance regulator, by boosting the efficacy of approved cystic fibrosis transmembrane conductance regulator modulators. Overall, this study unveils DRI-Pep #20 as a potent PI3Kγ/PKA disruptor for achieving therapeutic cAMP elevation in chronic respiratory disorders.
Keywords: AKAP; PI3Kγ; PKA; cAMP; peptide; respiratory diseases.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest Alessandra Ghigo and Emilio Hirsch are cofounders and shareholders of Kither Biotech Srl. Valentina Sala and Laura Tasca are employees of Kither Biotech Srl. All other authors report no conflicts of interest with the contents of the article.
Figures

References
-
- Murabito A., Cnudde S., Hirsch E., Ghigo A. Potential therapeutic applications of AKAP disrupting peptides. Clin. Sci. (Lond) 2020;134:3259–3282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

