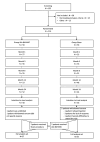
Allogenic bone marrow-derived mesenchymal stromal cell-based therapy for patients with chronic low back pain: a prospective, multicentre, randomised placebo controlled trial (RESPINE study)
- PMID: 39393844
- PMCID: PMC11503111
- DOI: 10.1136/ard-2024-225771
Allogenic bone marrow-derived mesenchymal stromal cell-based therapy for patients with chronic low back pain: a prospective, multicentre, randomised placebo controlled trial (RESPINE study)
Abstract
Objectives: To assess the efficacy of a single intradiscal injection of allogeneic bone marrow mesenchymal stromal cells (BM-MSCs) versus a sham placebo in patients with chronic low back pain (LBP).
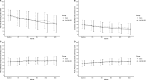
Methods: Participants were randomised in a prospective, double-blind, controlled study to receive either sham injection or intradiscal injection of 20 million allogeneic BM-MSC, between April 2018 and December 2022. The first co-primary endpoint was the rate of responders defined by improvement of the Visual Analogue Scale (VAS) for pain of at least 20% and 20 mm, or improvement of the Oswestry Disability Index (ODI) of 20% between baseline and month 12. The secondary structural co-primary endpoint was assessed by the disc fluid content measured by quantitative MRI T2, between baseline and month 12. Secondary endpoints included pain VAS, ODI, the Short Form (SF)-36 and the minimal clinically important difference in all timepoints (1, 3, 6, 12 and 24 months). We determined the immune response associated with allogeneic cell injection between baseline and 6 months. Serious adverse events (SAEs) were recorded.
Results: 114 patients were randomised (n=58, BM-MSC group; n=56, sham placebo group). At 12 months, the primary outcome was not reached (74% in the BM-MSC group vs 69% in the placebo group; p=0.77). The groups did not differ in all secondary outcomes. No SAE related to the intervention occurred.
Conclusions: While our study did not conclusively demonstrate the efficacy of allogeneic BM-MSCs for LBP, the procedure was safe. Long-term outcomes of MSC therapy for LBP are still being studied.
Trial registration number: EudraCT 2017-002092-25/ClinicalTrials.gov: NCT03737461.
Keywords: Biological Therapy; Low Back Pain; Orthopedic Procedures.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ on behalf of EULAR.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Ferreira ML, de Luca K, Haile LM. Global, regional, and national burden of low back pain, 1990-2020, its attributable risk factors, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5:e316–29. doi: 10.1016/S2665-9913(23)00098-X. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous