Zika viruses encode 5' upstream open reading frames affecting infection of human brain cells
- PMID: 39394194
- PMCID: PMC11470053
- DOI: 10.1038/s41467-024-53085-9
Zika viruses encode 5' upstream open reading frames affecting infection of human brain cells
Abstract
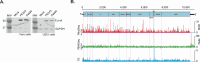
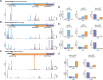
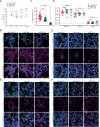
Zika virus (ZIKV), an emerging mosquito-borne flavivirus, is associated with congenital neurological complications. Here, we investigate potential pathological correlates of virus gene expression in representative ZIKV strains through RNA sequencing and ribosome profiling. In addition to the single long polyprotein found in all flaviviruses, we identify the translation of unrecognised upstream open reading frames (uORFs) in the genomic 5' region. In Asian/American strains, ribosomes translate uORF1 and uORF2, whereas in African strains, the two uORFs are fused into one (African uORF). We use reverse genetics to examine the impact on ZIKV fitness of different uORFs mutant viruses. We find that expression of the African uORF and the Asian/American uORF1 modulates virus growth and tropism in human cortical neurons and cerebral organoids, suggesting a potential role in neurotropism. Although the uORFs are expressed in mosquito cells, we do not see a measurable effect on transmission by the mosquito vector in vivo. The discovery of ZIKV uORFs sheds new light on the infection of the human brain cells by this virus and raises the question of their existence in other neurotropic flaviviruses.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Mlakar, J. et al. Zika virus associated with microcephaly. N. Engl. J. Med.374, 951–958 (2016). - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

