Nintedanib preserves lung growth and prevents pulmonary hypertension in a hyperoxia-induced lung injury model
- PMID: 39394424
- PMCID: PMC12119323
- DOI: 10.1038/s41390-024-03562-0
Nintedanib preserves lung growth and prevents pulmonary hypertension in a hyperoxia-induced lung injury model
Abstract
Background: Bronchopulmonary dysplasia (BPD), the chronic lung disease associated with prematurity, is characterized by poor alveolar and vascular growth, interstitial fibrosis, and pulmonary hypertension (PH). Although multifactorial in origin, the pathophysiology of BPD is partly attributed to hyperoxia-induced postnatal injury, resulting in lung fibrosis. Recent work has shown that anti-fibrotic agents, including Nintedanib (NTD), can preserve lung function in adults with idiopathic pulmonary fibrosis. However, NTD is a non-specific tyrosine kinase receptor inhibitor that can potentially have adverse effects on the developing lung, and whether NTD treatment can prevent or worsen risk for BPD and PH is unknown.
Hypothesis: We hypothesize that NTD treatment will preserve lung growth and function and prevent PH in an experimental model of hyperoxia-induced BPD in rats.
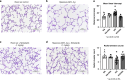
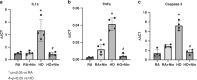
Methods: Newborn rats were exposed to either hyperoxia (90%) or room air (RA) conditions and received daily treatment of NTD or saline (control) by intraperitoneal (IP) injections (1 mg/kg) for 14 days, beginning on postnatal day 1. At day 14, lung mechanics were measured prior to harvesting lung and cardiac tissue. Lung mechanics, including total respiratory resistance and compliance, were measured using a flexiVent system. Lung tissue was evaluated for radial alveolar counts (RAC), mean linear intercept (MLI), pulmonary vessel density (PVD), and pulmonary vessel wall thickness (PVWT). Right ventricular hypertrophy (RVH) was quantified with cardiac weights using Fulton's index (ratio of right ventricle to the left ventricle plus septum).
Results: When compared with RA controls, hyperoxia exposure reduced RAC by 64% (p < 0.01) and PVD by 65% (p < 0.01) and increased MLI by 108% (p < 0.01) and RVH by 118% (p < 0.01). Hyperoxia increased total respiratory resistance by 94% and reduced lung compliance by 75% (p < 0.01 for each). NTD administration restored RAC, MLI, RVH, PVWT and total respiratory resistance to control values and improved PVD and total lung compliance in the hyperoxia-exposed rats. NTD treatment of control animals did not have adverse effects on lung structure or function at 1 mg/kg. When administered at higher doses of 50 mg/kg, NTD significantly reduced alveolar growth in RA controls, suggesting dose-related effects on normal lung structure.
Conclusions: We found that NTD treatment preserved lung alveolar and vascular growth, improved lung function, and reduced RVH in experimental BPD in infant rats without apparent adverse effects in control animals. We speculate that although potentially harmful at high doses, NTD may provide a novel therapeutic strategy for prevention of BPD and PH.
Impact: Anti-fibrotic therapies may be a novel therapeutic strategy for the treatment or prevention of BPD. High-dose anti-fibrotics may have adverse effects on developing lungs, while low-dose anti-fibrotics may treat or prevent BPD. There is very little preclinical and clinical data on the use of anti-fibrotics in the developing lung. Dose timing and duration of anti-fibrotic therapies may be critical for the treatment of neonatal lung disease. Currently, strategies for the prevention and treatment of BPD are lacking, especially in the context of lung fibrosis, so this research has major clinical applicability.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

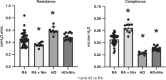



References
-
- Northway, W. H. Jr, Rosan, R. C. & Porter, D. Y. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N. Engl. J. Med.276, 357–368 (1967). - PubMed
-
- Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med.163, 1723–1729 (2001). - PubMed
-
- Horbar, J. D. et al. Trends in mortality and morbidities for infants born 24 to 28 weeks in the US: 1997-2021. Pediatrics153, e2023064153 (2024). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

