Selective activation of PPARα by pemafibrate mitigates peritoneal inflammation and fibrosis through suppression of NLRP3 inflammasome and modulation of inflammation
- PMID: 39394435
- PMCID: PMC11470028
- DOI: 10.1038/s41598-024-74340-5
Selective activation of PPARα by pemafibrate mitigates peritoneal inflammation and fibrosis through suppression of NLRP3 inflammasome and modulation of inflammation
Abstract
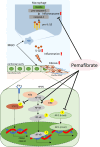
Peritoneal inflammation and fibrosis remain major challenges to the long-term maintenance of peritoneal dialysis. Pemafibrate, a selective peroxisome proliferator-activated receptor α (PPARα) modulator, has been implicated in the management of fibrosis-related disorders. We investigated whether pemafibrate ameliorates peritoneal inflammation and fibrosis and explored the underlying mechanisms in mice with methylglyoxal (MGO)-induced peritoneal fibrosis (MGO mice). MGO mice exhibited peritoneal fibrosis with increased expression of mesenchymal markers, transforming growth factor-β1 (TGF-β1), and substantial deposition of extracellular matrix (ECM) proteins. Additionally, MGO mice exhibited peritoneal inflammation as indicated by elevated tumor necrosis factor-α expression and macrophage infiltration in peritoneal tissue. These effects were mitigated by pemafibrate treatment, which also restored peritoneal membrane function. Furthermore, pemafibrate promoted anti-inflammatory macrophage polarization in both mice and THP-1 cells. In human peritoneal mesothelial cells (HPMCs), pemafibrate effectively inhibited interferon-γ-induced production of TGF-β1 and ECM while suppressing the proinflammatory cytokines nuclear factor-κB (NF-κB) and activator protein 1. The NF-κB inhibitory effect of pemafibrate involved stabilization of the NF-κB inhibitory protein IkBα. Notably, pemafibrate hindered activation of the NLR family pyrin domain containing 3/caspase-1 axis in interferon-γ-stimulated THP-1 cells. These findings suggest that pemafibrate ameliorates peritoneal inflammation and fibrosis, making it a promising candidate for peritoneal fibrosis therapy.
Keywords: Inflammasome; PPARα; Peritoneal dialysis; Peritoneal fibrosis; Peritoneal inflammation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Kawaguchi, Y. et al. Searching for the reasons for drop-out from peritoneal dialysis: A nationwide survey in Japan. Perit. Dial Int.23 (Suppl 2), S175–177 (2003). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

