Imipridones inhibit tumor growth and improve survival in an orthotopic liver metastasis mouse model of human uveal melanoma
- PMID: 39394450
- PMCID: PMC11589887
- DOI: 10.1038/s41416-024-02866-6
Imipridones inhibit tumor growth and improve survival in an orthotopic liver metastasis mouse model of human uveal melanoma
Abstract
Background: Uveal melanoma (UM) is a highly aggressive disease with very few treatment options. We previously demonstrated that mUM is characterized by high oxidative phosphorylation (OXPHOS). Here we tested the anti-tumor, signaling and metabolic effects of imipridones, which are CLPP activators, which inhibit OXPHOS indirectly and have demonstrated safety in patients.
Methods: We assessed CLPP expression in UM patient samples. We tested the effects of imipridones (ONC201 and ONC212) on the growth, survival, signaling and metabolism of UM cell lines in vitro, and for therapeutic efficacy in vivo in UM liver metastasis models.
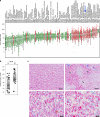
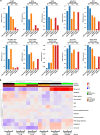
Results: CLPP expression was detected in primary and mUM patient samples. ONC201 and 212 decreased OXPHOS effectors, inhibited cell growth and migration, and induced apoptosis in human UM cell lines in vitro. ONC212 inhibited OXPHOS, increased metabolic stress and apoptotic pathways, inhibited amino acid metabolism, and induced cell death-related lipids. ONC212 also decreased tumor burden and increased survival in vivo in two UM liver metastasis models.
Conclusions: Imipridones are a promising strategy for further testing and development in mUM.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: VVP is an employee and stockholder of Chimerix/Oncoceutics. MAD has been a consultant to Roche/Genentech, Array, Pfizer, Novartis, BMS, GSK, Sanofi-Aventis, Vaccinex, Apexigen, Eisai, Iovance, Merck, and ABM Therapeutics, and he has been the PI of research grants to The University of Texas MD Anderson Cancer Center by Roche/Genentech, GSK, Sanofi-Aventis, Merck, Myriad, Oncothyreon, Pfizer, ABM Therapeutics, and LEAD Pharma. Ethics approval and consent to participate: All procedures performed in obtaining tissues from human participants were in accordance with the ethical standards of The University of Texas MD Anderson Cancer Center and ethical tenets in the Declaration of Helsinki. All the human tissues were obtained and banked under The University of Texas MD Anderson Cancer Center approved IRB protocol. We attest that all human tissues were obtained with informed consent from subjects involved. All animal studies and procedures were performed in accordance with The University of Texas at MD Anderson Cancer Center’s approved IACUC protocol following relevant guidelines and regulations. Consent for publication: All authors have been provided with a copy of the manuscript and have reviewed prior to submission and have provided consent to publication.
Figures




Update of
-
Imipridones inhibit tumor growth and improve survival in an orthotopic liver metastasis mouse model of human uveal melanoma.bioRxiv [Preprint]. 2024 Jan 15:2024.01.12.575058. doi: 10.1101/2024.01.12.575058. bioRxiv. 2024. Update in: Br J Cancer. 2024 Dec;131(11):1846-1857. doi: 10.1038/s41416-024-02866-6. PMID: 38293232 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

