Microbiome-derived metabolites in early to mid-pregnancy and risk of gestational diabetes: a metabolome-wide association study
- PMID: 39394552
- PMCID: PMC11470649
- DOI: 10.1186/s12916-024-03606-6
Microbiome-derived metabolites in early to mid-pregnancy and risk of gestational diabetes: a metabolome-wide association study
Abstract
Background: Pre-diagnostic disturbances in the microbiome-derived metabolome have been associated with an increased risk of diabetes in non-pregnant populations. However, the roles of microbiome-derived metabolites, the end-products of microbial metabolism, in gestational diabetes (GDM) remain understudied. We examined the prospective association of microbiome-derived metabolites in early to mid-pregnancy with GDM risk in a diverse population.
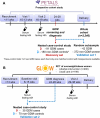
Methods: We conducted a prospective discovery and validation study, including a case-control sample of 91 GDM and 180 non-GDM individuals within the multi-racial/ethnic The Pregnancy Environment and Lifestyle Study (PETALS) as the discovery set, a random sample from the PETALS (42 GDM, 372 non-GDM) as validation set 1, and a case-control sample (35 GDM, 70 non-GDM) from the Gestational Weight Gain and Optimal Wellness randomized controlled trial as validation set 2. We measured untargeted fasting serum metabolomics at gestational weeks (GW) 10-13 and 16-19 by gas chromatography/time-of-flight mass spectrometry (TOF-MS), liquid chromatography (LC)/quadrupole TOF-MS, and hydrophilic interaction LC/quadrupole TOF-MS. GDM was diagnosed using the 3-h, 100-g oral glucose tolerance test according to the Carpenter-Coustan criteria around GW 24-28.
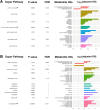
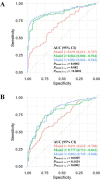
Results: Among 1362 annotated compounds, we identified 140 of gut microbiome metabolism origin. Multivariate enrichment analysis illustrated that carbocyclic acids and branched-chain amino acid clusters at GW 10-13 and the unsaturated fatty acids cluster at GW 16-19 were positively associated with GDM risk (FDR < 0.05). At GW 10-13, the prediction model that combined conventional risk factors and LASSO-selected microbiome-derived metabolites significantly outperformed the model with only conventional risk factors including fasting glucose (discovery AUC: 0.884 vs. 0.691; validation 1: 0.945 vs. 0.731; validation 2: 0.987 vs. 0.717; all P < 0.01). At GW 16-19, similar results were observed (discovery AUC: 0.802 vs. 0.691, P < 0.01; validation 1: 0.826 vs. 0.780; P = 0.10).
Conclusions: Dysbiosis in microbiome-derived metabolites is present early in pregnancy among individuals progressing to GDM.
Keywords: Gestational diabetes; Metabolomics; Microbiome; Pregnancy; Risk prediction.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures