Deciphering the role of SAMHD1 in endometrial cancer progression
- PMID: 39394602
- PMCID: PMC11468744
- DOI: 10.1186/s13062-024-00525-7
Deciphering the role of SAMHD1 in endometrial cancer progression
Abstract
Background: Endometrial cancer (EC) presents significant clinical challenges due to its heterogeneity and complex pathophysiology. SAMHD1, known for its role as a deoxynucleotide triphosphate triphosphohydrolase, has been implicated in the progression of various cancers, including EC. This study focuses on elucidating the role of SAMHD1 in EC through its impact on TRIM27-mediated PTEN ubiquitination.
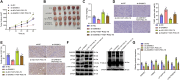
Results: Utilizing a combination of bioinformatics and cellular biology techniques, we investigated the interactions among SAMHD1, TRIM27, and PTEN. Our findings reveal that SAMHD1 modulates PTEN ubiquitination via TRIM27, impacting key pathways involved in EC pathogenesis. These interactions suggest a critical mechanism by which SAMHD1 could influence tumor behavior and progression in EC.
Conclusions: The results from this study underscore the potential of targeting the SAMHD1-TRIM27-PTEN axis as a therapeutic strategy in EC. By providing new insights into the molecular mechanisms underlying EC progression, our research supports the development of novel therapeutic approaches that could contribute to improve treatment strategies for patients with EC.
Keywords: PI3K/AKT signaling pathway; PTEN; SAMHD1; TRIM27; Ubiquitination; dNTP hydrolysis enzyme.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366(9484):491–505. 10.1016/S0140-6736(05)67063-8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

