Cancer cell-selective induction of mitochondrial stress and immunogenic cell death by PT-112 in human prostate cell lines
- PMID: 39394618
- PMCID: PMC11470694
- DOI: 10.1186/s12967-024-05739-x
Cancer cell-selective induction of mitochondrial stress and immunogenic cell death by PT-112 in human prostate cell lines
Abstract
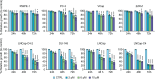
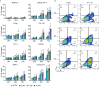
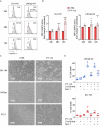
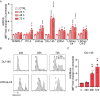
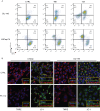
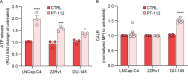
PT-112 is a novel immunogenic cell death (ICD)-inducing small molecule currently under Phase 2 clinical development, including in metastatic castration-resistant prostate cancer (mCRPC), an immunologically cold and heterogeneous disease state in need of novel therapeutic approaches. PT-112 has been shown to cause ribosome biogenesis inhibition and organelle stress followed by ICD in cancer cells, culminating in anticancer immunity. In addition, clinical evidence of PT-112-driven immune effects has been observed in patient immunoprofiling. Given the unmet need for immune-based therapies in prostate cancer, along with a Phase I study (NCT#02266745) showing PT-112 activity in mCRPC patients, we investigated PT-112 effects in a panel of human prostate cancer cell lines. PT-112 demonstrated cancer cell selectivity, inhibiting cell growth and leading to cell death in prostate cancer cells without affecting the non-tumorigenic epithelial prostate cell line RWPE-1 at the concentrations tested. PT-112 also caused caspase-3 activation, as well as stress features in mitochondria including ROS generation, compromised membrane integrity, altered respiration, and morphological changes. Moreover, PT-112 induced damage-associated molecular pattern (DAMP) release, the first demonstration of ICD in human cancer cell lines, in addition to autophagy initiation across the panel. Taken together, PT-112 caused selective stress, growth inhibition and death in human prostate cancer cell lines. Our data provide additional insight into mitochondrial stress and ICD in response to PT-112. PT-112 anticancer immunogenicity could have clinical applications and is currently under investigation in a Phase 2 mCRPC study.
Keywords: Autophagy; Immunogenic cell death; Mitochondrial stress; PT-112; Prostate cancer.
© 2024. The Author(s).
Conflict of interest statement
C.Y. Yim, M. Congenie, T.D. Ames, M.R. Price and J. Jimeno are or have been employees of Promontory Therapeutics Inc.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

