Leukocytes Do Not Influence the Safety and Efficacy of Platelet-Rich Plasma Injections for the Treatment of Knee Osteoarthritis: A Double-Blind Randomized Controlled Trial
- PMID: 39394763
- PMCID: PMC11542329
- DOI: 10.1177/03635465241283500
Leukocytes Do Not Influence the Safety and Efficacy of Platelet-Rich Plasma Injections for the Treatment of Knee Osteoarthritis: A Double-Blind Randomized Controlled Trial
Abstract
Background: Platelet-rich plasma (PRP) is increasingly used for the injection treatment of knee osteoarthritis (OA). However, the role of leukocytes contained in PRP is controversial, with some preclinical studies suggesting detrimental effects and others emphasizing their contribution in secreting bioactive molecules.
Purpose: To compare the safety and effectiveness of leukocyte-rich PRP (LR-PRP) and leukocyte-poor PRP (LP-PRP) for the treatment of knee OA.
Hypothesis: That leukocytes could influence results both in terms of adverse events and clinical outcomes.
Study design: Randomized controlled trial; Level of evidence, 1.
Methods: This double-blind randomized controlled trial included 132 patients with Kellgren-Lawrence grade 1-3 knee OA who were randomized to a 3-injection cycle of either LR-PRP or LP-PRP. Patients were prospectively assessed at baseline and at 2, 6, and 12 months with subjective evaluations comprising the International Knee Documentation Committee (IKDC) subjective score, the KOOS (Knee injury and Osteoarthritis Outcome Score), the WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index), the visual analog scale for pain, the EuroQol-visual analog scale, the EuroQol-5 dimensions, and the Tegner activity scale. Objective evaluations consisted of the IKDC objective score, active/passive range of motion, and circumference of the index and contralateral knees. Patient judgment of the treatment was recorded as well as adverse reactions and failures.
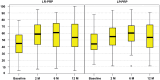
Results: An overall improvement in subjective and objective outcomes was documented, with no differences between the 2 groups, except for the improvement in the IKDC subjective score at 2 months, which was greater for the LR-PRP group compared with the LP-PRP group (14.8 ± 14.8 vs 8.6 ± 13.3, respectively; P = .046), as well as for active (P = .021) and passive (P = .040) ROM of the index knee at 6 months, showing statistically significant higher values in the LP-PRP group; and for quadriceps circumference of the index (P = .042) and contralateral (P = .045) knees at 12 months, which were significantly greater in the LR-PRP group. The IKDC subjective score improved from 42.5 ± 17.6 at baseline to 55.6 ± 21.4 at 12 months for the LR-PRP group (P < .0005) and from 45.7 ± 16.4 to 55.3 ± 20.4 for the LP-PRP group (P = .001). No differences in terms of patient treatment judgment were observed at all follow-up time points. No severe adverse events related to the treatment were reported, but some mild adverse events related to the treatment were observed: 16 in the LR-PRP group and 17 in the LP-PRP group. Treatment failed in 5 patients in the LR-PRP group and 2 in the LP-PRP group.
Conclusion: This double-blind randomized controlled trial demonstrated that leukocytes did not affect the safety and efficacy of intra-articular PRP injections for the treatment of patients with knee OA. Both LR-PRP and LP-PRP demonstrated comparable clinical outcomes at all follow-up time points, without showing differences in subjective and objective outcomes or in adverse events and treatment failures.
Registration: NCT04187183 (ClinicalTrials.gov).
Keywords: injection; intra-articular; knee; leukocytes; osteoarthritis; platelet-rich plasma (PRP).
Conflict of interest statement
One or more of the authors has declared the following potential conflict of interest or source of funding: S.Z. has received institutional support from Fidia Farmaceutici, CartiHeal, IGEA Clinical Biophysics, Biomet, and Kensey Nash; grant support from I+; and royalties from Springer outside the submitted work. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Figures
References
-
- Abbas A, Du JT, Dhotar HS. The effect of leukocyte concentration on platelet-rich plasma injections for knee osteoarthritis: a network meta-analysis. J Bone Joint Surg Am. 2022;104(6):559-570. - PubMed
-
- Bensa A, Albanese J, Boffa A, Previtali D, Filardo G. Intra-articular corticosteroid injections provide a clinically relevant benefit compared to placebo only at short-term follow-up in patients with knee osteoarthritis: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2024;32(2):311-322. - PubMed
-
- Bensa A, Previtali D, Boffa A, Salerno M, Sangiorgio A, Filardo G. PRP injections for the treatment of knee osteoarthritis: the improvement is clinically significant only with high-platelet PRP. A meta-analysis of randomized controlled trials. Am J Sports Med. In press. doi: 10.1177/03635465241246524.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

