Toward Understanding the Mechanism of Client-Selective Small Molecule Inhibitors of the Sec61 Translocon
- PMID: 39394908
- PMCID: PMC11695074
- DOI: 10.1002/jmr.3108
Toward Understanding the Mechanism of Client-Selective Small Molecule Inhibitors of the Sec61 Translocon
Erratum in
-
Correction to "Toward Understanding the Mechanism of Client-Selective Small Molecule Inhibitors of the Sec61 Translocon".J Mol Recognit. 2025 Sep;38(5):e70010. doi: 10.1002/jmr.70010. J Mol Recognit. 2025. PMID: 40769512 Free PMC article. No abstract available.
Abstract
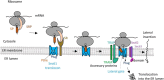
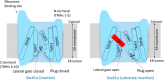
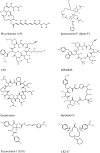
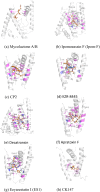
The Sec61 translocon mediates the translocation of numerous, newly synthesized precursor proteins into the lumen of the endoplasmic reticulum or their integration into its membrane. Recently, structural biology revealed conformations of idle or substrate-engaged Sec61, and likewise its interactions with the accessory membrane proteins Sec62, Sec63, and TRAP, respectively. Several natural and synthetic small molecules have been shown to block Sec61-mediated protein translocation. Since this is a key step in protein biogenesis, broad inhibition is generally cytotoxic, which may be problematic for a putative drug target. Interestingly, several compounds exhibit client-selective modes of action, such that only translocation of certain precursor proteins was affected. Here, we discuss recent advances of structural biology, molecular modelling, and molecular screening that aim to use Sec61 as feasible drug target.
© 2024 The Author(s). Journal of Molecular Recognition published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Osborne A. R., Rapoport T. A., and van den Berg B., “Protein Translocation by the Sec61/SecY Channel,” Annual Review of Cell and Developmental Biology 21 (2005): 529–550. - PubMed
-
- Corsi A. K. and Schekman R., “Mechanism of Polypeptide Translocation Into the Endoplasmic Reticulum,” Journal of Biological Chemistry 271 (1996): 30299–30302. - PubMed
-
- Palade G., “Intracellular Aspects of Protein Synthesis,” Science 189 (1975): 347–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

