Reticulophagy and viral infection
- PMID: 39394962
- PMCID: PMC11702952
- DOI: 10.1080/15548627.2024.2414424
Reticulophagy and viral infection
Abstract
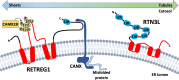
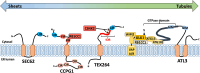
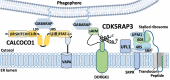
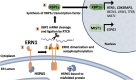
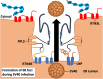
All viruses are obligate intracellular parasites that use host machinery to synthesize viral proteins. In infected eukaryotes, viral secreted and transmembrane proteins are synthesized at the endoplasmic reticulum (ER). Many viruses refashion ER membranes into bespoke factories where viral products accumulate while evading host pattern recognition receptors. ER processes are tightly regulated to maintain cellular homeostasis, so viruses must either conform to ER regulatory mechanisms or subvert them to ensure efficient viral replication. Reticulophagy is a catabolic process that directs lysosomal degradation of ER components. There is accumulating evidence that reticulophagy serves as a form of antiviral defense; we call this defense "xERophagy" to acknowledge its relationship to xenophagy, the catabolic degradation of microorganisms by macroautophagy/autophagy. In turn, viruses can subvert reticulophagy to suppress host antiviral responses and support efficient viral replication. Here, we review the evidence for functional interplay between viruses and the host reticulophagy machinery.Abbreviations: AMFR: autocrine motility factor receptor; ARF4: ADP-ribosylation factor 4; ARL6IP1: ADP-ribosylation factor-like 6 interacting protein 1; ATL3: atlastin GTPase 3; ATF4: activating transcription factor 4; ATF6: activating transcription factor 6; BPIFB3: BPI fold containing family B, member 3; CALCOCO1: calcium binding and coiled coil domain 1; CAMK2B: calcium/calmodulin-dependent protein kinase II, beta; CANX: calnexin; CDV: canine distemper virus; CCPG1: cell cycle progression 1; CDK5RAP3/C53: CDK5 regulatory subunit associated protein 3; CIR: cargo-interacting region; CoV: coronavirus; CSNK2/CK2: casein kinase 2; CVB3: coxsackievirus B3; DAPK1: death associated protein kinase 1; DENV: dengue virus; DMV: double-membrane vesicles; EBOV: Ebola virus; EBV: Epstein-Barr Virus; EIF2AK3/PERK: eukaryotic translation initiation factor 2 alpha kinase 3; EMCV: encephalomyocarditis virus; EMV: extracellular microvesicle; ER: endoplasmic reticulum; ERAD: ER-associated degradation; ERN1/IRE1: endoplasmic reticulum to nucleus signalling 1; EV: extracellular vesicle; EV71: enterovirus 71; FIR: RB1CC1/FIP200-interacting region; FMDV: foot-and-mouth disease virus; HCMV: human cytomegalovirus; HCV: hepatitis C virus; HMGB1: high mobility group box 1; HSPA5/BiP: heat shock protein 5; IFN: interferon; IFNG/IFN-γ: interferon gamma; KSHV: Kaposi's sarcoma-associated herpesvirus; LIR: MAP1LC3/LC3-interacting region; LNP: lunapark, ER junction formation factor; MAP1LC3: microtubule-associated protein 1 light chain 3; MAP3K5/ASK1: mitogen-activated protein kinase kinase kinase 5; MAPK/JNK: mitogen-activated protein kinase; MeV: measles virus; MHV: murine hepatitis virus; NS: non-structural; PDIA3: protein disulfide isomerase associated 3; PRR: pattern recognition receptor; PRRSV: porcine reproductive and respiratory syndrome virus; RB1CC1/FIP200: RB1-inducible coiled-coil 1; RETREG1/FAM134B: reticulophagy regulator 1; RHD: reticulon homology domain; RTN3: reticulon 3; RTN3L: reticulon 3 long; sAIMs: shuffled Atg8-interacting motifs; SARS-CoV: severe acute respiratory syndrome coronavirus; SINV: Sindbis virus; STING1: stimulator of interferon response cGAMP interactor 1; SVV: Seneca Valley virus; SV40: simian virus 40; TEX264: testis expressed gene 264 ER-phagy receptor; TFEB: transcription factor EB; TRAF2: TNF receptor-associated factor 2; UIM: ubiquitin-interacting motif; UFM1: ubiquitin-fold modifier 1; UPR: unfolded protein response; VAPA: vesicle-associated membrane protein, associated protein A; VAPB: vesicle-associated membrane protein, associated protein B and C; VZV: varicella zoster virus; WNV: West Nile virus; XBP1: X-box binding protein 1; XBP1s: XBP1 spliced; xERophagy: xenophagy involving reticulophagy; ZIKV: Zika virus.
Keywords: Autophagy; endoplasmic reticulum; reticulophagy; unfolded protein response; virus.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
