Development of a Physiologically Based Pharmacokinetic (PBPK) Model for F-53B in Pregnant Mice and Its Extrapolation to Humans
- PMID: 39394996
- PMCID: PMC11500426
- DOI: 10.1021/acs.est.4c05405
Development of a Physiologically Based Pharmacokinetic (PBPK) Model for F-53B in Pregnant Mice and Its Extrapolation to Humans
Abstract
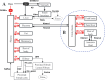
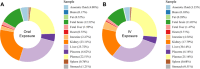
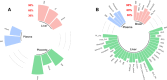
Chlorinated polyfluorinated ether sulfonic acid (F-53B), a commonly utilized alternative for perfluorooctane sulfonate, was detected in pregnant women and cord blood recently. However, the lack of detailed toxicokinetic information poses a significant challenge in assessing the human risk assessment for F-53B exposure. Our study aimed to develop a physiologically based pharmacokinetic (PBPK) model for pregnant mice, based on toxicokinetic experiments, and extrapolating it to humans. Pregnant mice were administered 80 μg/kg F-53B orally and intravenously on gestational day 13. F-53B concentrations in biological samples were analyzed via ultraperformance liquid chromatography-mass spectrometry. Results showed the highest F-53B accumulation in the brain, followed by the placenta, amniotic fluid, and liver in fetal mice. These toxicokinetic data were applied to F-53B PBPK model development and evaluation, and Monte Carlo simulations were used to characterize the variability and uncertainty in the human population. Most of the predictive values were within a 2-fold range of experimental data (>72%) and had a coefficient of determination (R2) greater than 0.68. The developed mouse model was then extrapolated to the human and evaluated with human biomonitoring data. Our study provides an important step toward improving the understanding of toxicokinetics of F-53B and enhancing the quantitative risk assessments in sensitive populations, particularly in pregnant women and fetuses.
Keywords: F-53B; Monte Carlo (MC) simulation; perfluorooctanesulfonate (PFOS); physiologically based pharmacokinetic (PBPK) modeling.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Wang S.; Huang J.; Yang Y.; Hui Y.; Ge Y.; Larssen T.; Yu G.; Deng S.; Wang B.; Harman C. First Report of a Chinese PFOS Alternative Overlooked for 30 Years: Its Toxicity, Persistence, and Presence in the Environment. Environ. Sci. Technol. 2013, 47 (18), 10163–10170. 10.1021/es401525n. - DOI - PubMed
-
- Liu L.-S.; Guo Y.-T.; Wu Q.-Z.; Zeeshan M.; Qin S.-J.; Zeng H.-X.; Lin L.-Z.; Chou W.-C.; Yu Y.-J.; Dong G.-H.; et al. Per- and Polyfluoroalkyl Substances in Ambient Fine Particulate Matter in the Pearl River Delta, China: Levels, Distribution and Health Implications. Environ. Pollut. 2023, 334, 122138. 10.1016/j.envpol.2023.122138. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

