Normalization of short-chain fatty acid concentration by bacterial count of stool samples improves discrimination between eubiotic and dysbiotic gut microbiota caused by Clostridioides difficile infection
- PMID: 39395000
- PMCID: PMC11485779
- DOI: 10.1080/19490976.2024.2415488
Normalization of short-chain fatty acid concentration by bacterial count of stool samples improves discrimination between eubiotic and dysbiotic gut microbiota caused by Clostridioides difficile infection
Abstract
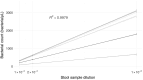
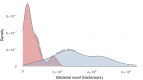
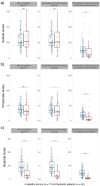
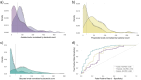
Short-chain fatty acids (SCFAs) represent a cornerstone of gut health, serving as critical mediators of immune modulation and overall host homeostasis. Patients with dysbiosis caused by Clostridioides difficile infection (CDI) typically exhibit lower SCFAs levels compared to healthy stool donors and, thus, the concentration of SCFAs has been proposed as a proxy marker of a healthy microbiota. However, there is no consistency in the methods used to quantify SCFAs in stool samples and usually, the results are normalized by the weight of the stool samples, which does not address differences in water and fiber content and ignores bacterial counts in the sample (the main component of stool that contributes to the composition of these metabolites in the sample). Here, we show that normalized SCFAs concentrations by the bacterial count improve discrimination between healthy and dysbiotic samples (patients with CDI), particularly when using acetate and propionate levels. After normalization, butyrate is the metabolite that best discriminates eubiotic and dysbiotic samples according to the area under the receiver operating characteristic (ROC) curve (AUC-ROC = 0.860, [95% CI: 0.786-0.934], p < .0001).
Keywords: Clostridioides difficile; Short-chain fatty acids (SCFAs); dysbiosis; fecal microbiota transplantation (FMT); gut; intestinal microbiota.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures
Similar articles
-
Gut microbiome and plasma lipidome analysis reveals a specific impact of Clostridioides difficile infection on intestinal bacterial communities and sterol metabolism.mBio. 2024 Oct 16;15(10):e0134724. doi: 10.1128/mbio.01347-24. Epub 2024 Aug 27. mBio. 2024. PMID: 39189787 Free PMC article.
-
Butyrate Differentiates Permissiveness to Clostridioides difficile Infection and Influences Growth of Diverse C. difficile Isolates.Infect Immun. 2023 Feb 16;91(2):e0057022. doi: 10.1128/iai.00570-22. Epub 2023 Jan 24. Infect Immun. 2023. PMID: 36692308 Free PMC article.
-
Characterization and dynamics of intestinal microbiota in patients with Clostridioides difficile colonization and infection.Microbes Infect. 2024 Nov-Dec;26(8):105373. doi: 10.1016/j.micinf.2024.105373. Epub 2024 Jun 8. Microbes Infect. 2024. PMID: 38857786
-
Understanding the mechanisms of efficacy of fecal microbiota transplant in treating recurrent Clostridioides difficile infection and beyond: the contribution of gut microbial-derived metabolites.Gut Microbes. 2020 Nov 9;12(1):1810531. doi: 10.1080/19490976.2020.1810531. Gut Microbes. 2020. PMID: 32893721 Free PMC article. Review.
-
A short chain fatty acid-centric view of Clostridioides difficile pathogenesis.PLoS Pathog. 2021 Oct 21;17(10):e1009959. doi: 10.1371/journal.ppat.1009959. eCollection 2021 Oct. PLoS Pathog. 2021. PMID: 34673840 Free PMC article. Review.
Cited by
-
Fecal Microbiota Transplantation (FMT) in Clostridium difficile Infection: A Paradigm Shift in Gastrointestinal Microbiome Modulation.Cureus. 2025 May 29;17(5):e85054. doi: 10.7759/cureus.85054. eCollection 2025 May. Cureus. 2025. PMID: 40585700 Free PMC article.
-
The Effect of Microbiome-Derived Metabolites in Inflammation-Related Cancer Prevention and Treatment.Biomolecules. 2025 May 8;15(5):688. doi: 10.3390/biom15050688. Biomolecules. 2025. PMID: 40427581 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
