Phase 2 study of the antitumour activity and safety of simlukafusp alfa (FAP-IL2v) combined with atezolizumab in patients with recurrent and/or metastatic cervical squamous cell carcinoma
- PMID: 39395231
- PMCID: PMC11663756
- DOI: 10.1016/j.ebiom.2024.105374
Phase 2 study of the antitumour activity and safety of simlukafusp alfa (FAP-IL2v) combined with atezolizumab in patients with recurrent and/or metastatic cervical squamous cell carcinoma
Abstract
Background: Simlukafusp alfa (FAP-IL2v) is an immune cytokine engineered to selectively promote immune responses in the tumour microenvironment. We evaluated the antitumour activity and safety of FAP-IL2v plus atezolizumab in recurrent and/or metastatic cervical squamous cell carcinoma (SCC) in a phase 2 basket study (NCT03386721).
Methods: Patients with confirmed metastatic, persistent or recurrent cervical SCC who had progressed on ≥1 anti-cancer therapy and had measurable disease were enrolled. FAP-IL2v 10 mg was administered once every 3 weeks (Q3W) or once weekly (QW) for 4 weeks then once every 2 weeks (Q2W) with the corresponding Q3W or Q2W atezolizumab regimens. The primary endpoint was objective response rate by investigator assessment.
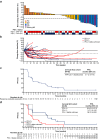
Findings: Forty-eight patients were enrolled (Q3W: n = 47; QW/Q2W: n = 1). Among 45 response evaluable patients, objective responses occurred in 12 patients (27%; CI 16.0-41.0), including 3 complete and 9 partial responses. Responses occurred in 6/19 PD-L1 positive patients (32%; 95% CI 15.4-54.0) and 5/24 PD-L1 negative patients (21%; 95% CI 9.2-35.6). Median duration of response was 13.3 months (95% CI 7.6-NE). Median progression-free survival was 3.7 months (95% CI 3.3-9.0). Adverse events (AEs) were consistent with the known safety profile of each drug. AEs leading to withdrawal of either agent occurred in 6 patients (13%). Pronounced expansion and activation of natural killer and CD8 T cells in peripheral blood and increased tumour infiltration and inflammation were observed.
Interpretation: FAP-IL2v plus atezolizumab is clinically active and has manageable safety in patients with recurrent and/or metastatic cervical SCC.
Funding: F. Hoffmann-La Roche Ltd.
Keywords: Cervical cancer; IL-2; Immunotherapy; PD-L1; Squamous cell carcinoma.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests L. Verlingue reports grants from Bristol-Myers Squibb; royalties or licenses from RESOLVED; payment or honoraria from Bristol-Myers Squibb; leadership or fiduciary role as CEO of RESOLVED; stock or stock options from RESOLVED; as part of the Drug Development Department (DITEP) of Gustave Roussy and Early Phase Unit of Centre Léon Bérard, L. Verlingue reports being Principal/Sub-Investigator of clinical trials for AbbVie, Adaptimmune, Aduro Biotech, Agios Pharmaceuticals, Amgen, argenx, Arno Therapeutics, Astex Pharmaceuticals, AstraZeneca, AVEO pharmaceuticals, Basilea Pharmaceutica, Bayer, BBB Technologies, BeiGene, Blueprint Medicines, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol-Myers Squibb, Celgene Corporation, Chugai Pharmaceutical Co., Clovis Oncology, Cullinan-Apollo, Daiichi Sankyo, Debiopharm, Eisai, Eli Lilly, Exelixis, Faron Pharmaceuticals, F. Hoffmann-La Roche, Forma Therapeutics, GamaMabs Pharma, Genentech, GlaxoSmithKline, H3 Biomedicine, ImCheck Therapeutics, Innate Pharma, Institut De Recherche Pierre Fabre, Iris Servier, Janssen Pharmaceuticals, Kura Oncology, Kyowa Kirin, Loxo Oncology, Lytix Biopharma, Medimmune, Menarini Ricerche, Merck Sharpe & Dohme, Merrimack Pharmaceuticals, Merus, Millennium Pharmaceuticals, Molecular Partners, Nanobiotix, Nektar Therapeutics, Novartis, Octimet Oncology, Oncoethix, Oncopeptides, Orion Pharma, Ose Pharma, Pfizer, PharmaMar, Pierre Fabre, Medicament, Sanofi, Seattle Genetics, SOTIO, Servier, Syros Pharmaceuticals, Taiho Pharma, Tesaro, and Xencor; research grants from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen Pharmaceuticals, Merck Sharpe & Dohme, Novartis, Onxeo, Pfizer, Roche, and Sanofi; non-financial support (drug supplied) from AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Medimmune, Merck Sharpe & Dohme, NH TherAGuiX, Onxeo, and Pfizer. H. Prenen reports payment or honoraria for presentations from Amgen, AstraZeneca, Bayer, Roche, and Sanofi; support for attending meetings from AstraZeneca, Bayer, and Roche; personal fees for participation on a Data Safety Monitoring Board or Advisory Board for Biocartis and Cureteq. E. M. Guerra Alia reports personal consulting fees for AstraZeneca, Clovis Oncology, GlaxoSmithKline, Merck Sharpe & Dohme, PharmaMar, and Roche; payment or honoraria from AstraZeneca (personal), Clovis Oncology, GlaxoSmithKline, Merck Sharpe & Dohme, and PharmaMar; personal payment for expert testimony from AstraZeneca, Clovis Oncology, GlaxoSmithKline, Merck Sharpe & Dohme, PharmaMar, and Roche; personal support for attending meetings and/or travel from AstraZeneca and GlaxoSmithKline. R. Perets reports personal consulting fees for 1Etx, Galmed Therapeutics, and Gilboa Therapeutics; payment or honoraria from Merck Sharpe & Dohme; support for attending meetings and/or travel from Pfizer. I Lugowska reports grants or contracts from Agenus and Roche; personal payment or honoraria from Bristol-Myers Squibb, Novartis, and Merck Sharpe & Dohme; support for attending a meeting and/or travel from ESMO; other financial interests or non-financial interests from Clinnote. A. Taus reports payment or honoraria from AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck Sharpe & Dohme, Pfizer, Roche, Sanofi, and Takeda. A. Oaknin reports institutional funding from AbbVie, Advaxis, Aeterna Zentaris, Amgen, Aprea Therapeutics, Bristol-Myers Squibb, Clovis Oncology, Eisai, Immunogen, Merck Sharpe & Dohme, Millennium Pharmaceuticals, PharmaMar, Regeneron Pharmaceuticals, Roche, and Tesaro; personal fees for participation on an Advisory Board from Agenus, AstraZeneca, Clovis Oncology, Corcept Therapeutics, Deciphera Pharmaceuticals, Eisai, EMD Serono, Genmab, GlaxoSmithKline, ImmunoGen, Itheos, Merck Sharpe & Dohme, Mersana Therapeutics, Novocure, OneXerna Therapeutics, PharmaMar, Regeneron, Roche, Sattucklabs, Seagen, and Sutro Biopharm. S. Goksu reports institutional payment or honoraria from Bristol-Myers Squibb, Merck Sharpe & Dohme, Novartis, and Pfizer; participation on a Data Safety Monitoring Board or Advisory Board from Novartis and Pfizer. S. Roselló-Keränen reports personal payment or honoraria for presentations from Amgen, Merck Sharpe & Dohme, and Servier; personal fees for participation on an Advisory Board from Pierre Fabre and Sirtex. R. Dziadziuszko reports personal consulting fees from AstraZeneca, Bristol-Myers Squibb, Merck Sharpe & Dohme, Pfizer, Novartis, Roche, and Takeda; payment or honoraria from AstraZeneca, Amgen, Pfizer, Novartis, Roche, and Takeda; support for attending meetings and or travel from Pfizer; receipt of equipment, materials, drugs, medical writing, gifts, or other services from Novartis and Pfizer. C. Habigt is a Roche employee with stock options. D. Marbach is a Roche employee. C. Boetsch is a Roche employee with stock options. D. Dejardin is a Roche employee with patents/stocks. N. Sleiman is a Roche employee. S. Evers is a former Roche employee and current shareholder. M. Richard is a Roche employee with stock options. C. Ardeshir is a Roche employee and shareholder. J. Charo is a Roche employee with patents/stocks. A. Kraxner is a Roche employee with stocks and stock options. V. Teichgräber is a Roche employee with stock options. N. Keshelava is a Roche employee with stock options. A. Italiano, D. Tosi, V. Moiseyenko, M. Gumus, C. Arslan, C. Lindsay, S. Deva, S. Rottey, I. Cicin, and A. Smolin have no disclosures to report.
Figures

References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- National Cancer Institute Cervical cancer treatment (PDQ®) – health professional version 2022. https://www.cancer.gov/types/cervical/hp/cervical-treatment-pdq (accessed December 2023)
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

