Roadmap on magnetic nanoparticles in nanomedicine
- PMID: 39395441
- PMCID: PMC11539342
- DOI: 10.1088/1361-6528/ad8626
Roadmap on magnetic nanoparticles in nanomedicine
Abstract
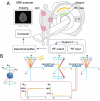
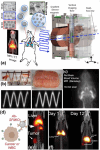
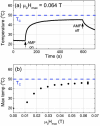
Magnetic nanoparticles (MNPs) represent a class of small particles typically with diameters ranging from 1 to 100 nanometers. These nanoparticles are composed of magnetic materials such as iron, cobalt, nickel, or their alloys. The nanoscale size of MNPs gives them unique physicochemical (physical and chemical) properties not found in their bulk counterparts. Their versatile nature and unique magnetic behavior make them valuable in a wide range of scientific, medical, and technological fields. Over the past decade, there has been a significant surge in MNP-based applications spanning biomedical uses, environmental remediation, data storage, energy storage, and catalysis. Given their magnetic nature and small size, MNPs can be manipulated and guided using external magnetic fields. This characteristic is harnessed in biomedical applications, where these nanoparticles can be directed to specific targets in the body for imaging, drug delivery, or hyperthermia treatment. Herein, this roadmap offers an overview of the current status, challenges, and advancements in various facets of MNPs. It covers magnetic properties, synthesis, functionalization, characterization, and biomedical applications such as sample enrichment, bioassays, imaging, hyperthermia, neuromodulation, tissue engineering, and drug/gene delivery. However, as MNPs are increasingly explored forin vivoapplications, concerns have emerged regarding their cytotoxicity, cellular uptake, and degradation, prompting attention from both researchers and clinicians. This roadmap aims to provide a comprehensive perspective on the evolving landscape of MNP research.
Keywords: biomedical application; drug delivery; hyperthermia; magnetic biosensing; magnetic imaging; magnetic nanoparticle; tissue engineering.
Creative Commons Attribution license.
Figures
















References
-
- Kittel C. Introduction to Solid State Physics. Wiley; 2018.
-
- Ramos-Guivar J A, Lopez E O, Greneche J-M, Litterst F J, Passamani E C. Effect of EDTA organic coating on the spin canting behavior of maghemite nanoparticles for lead (II) adsorption. Appl. Surf. Sci. 2021;538:148021. doi: 10.1016/j.apsusc.2020.148021. - DOI
-
- Pereira A M, Pereira C, Silva A S, Schmool D S, Freire C, Greneche J-M, Araujo J P. Unravelling the effect of interparticle interactions and surface spin canting in γ-Fe2O3@ SiO2 superparamagnetic nanoparticles. J. Appl. Phys. 2011;109:114319. doi: 10.1063/1.3583652. - DOI
-
- Yari P, Chugh V K, Saha R, Tonini D, Rezaei B, Mostufa S, Xu K, Wang J-P, Wu K. Static and dynamic magnetization models of magnetic nanoparticles: an appraisal. Phys. Scr. 2023;98:082002. doi: 10.1088/1402-4896/ace8d1. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
