Site-specific O-GlcNAcylation of progesterone receptor (PR) supports PR attenuation of interferon stimulated genes (ISGs) and tumor growth in breast cancer
- PMID: 39395796
- PMCID: PMC11609360
- DOI: 10.1016/j.jbc.2024.107886
Site-specific O-GlcNAcylation of progesterone receptor (PR) supports PR attenuation of interferon stimulated genes (ISGs) and tumor growth in breast cancer
Abstract
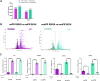
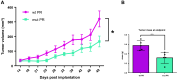
Hormone receptor positive (HR+) breast cancer, defined by expression of estrogen receptor (ER) and/or progesterone receptor (PR), is the most commonly diagnosed type of breast cancer. PR alters the transcriptional landscape to support tumor growth in concert with, or independent of, ER. Understanding the mechanisms regulating PR function is critical to developing new strategies to treat HR+ breast cancer. O-linked β-N-acetylglucosamine (O-GlcNAc) is a posttranslational modification responsible for nutrient sensing that modulates protein function. Although PR is heavily posttranslationally modified, through both phosphorylation and O-GlcNAcylation, specific sites of O-GlcNAcylation on PR and how they regulate PR action have not been investigated. Using established PR-expressing breast cancer cell lines, we mapped several sites of O-GlcNAcylation on PR. RNA-sequencing after PR O-GlcNAc site mutagenesis revealed site-specific O-GlcNAcylation of PR is critical for ligand-independent suppression of interferon signaling, a regulatory function of PR in breast cancer. Furthermore, O-GlcNAcylation of PR enhances PR-driven tumor growth in vivo. Herein, we have delineated one contributing mechanism to PR function in breast cancer that impacts tumor growth and provided additional insight into the mechanism through which PR attenuates interferon signaling.
Keywords: O-GlcNAc; breast; cancer; interferon; progesterone.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflict of interest with the contents of this article.
Figures



References
-
- Beral V. Breast cancer and hormone-replacement therapy in the million women study. Lancet. 2003;362:419–427. - PubMed
-
- Anderson G.L., Limacher M., Assaf A.R., Bassford T., Beresford S.A., Black H., et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

