Neuronal splicing of the unmethylated histone H3K4 reader, PHF21A, prevents excessive synaptogenesis
- PMID: 39395799
- PMCID: PMC11605454
- DOI: 10.1016/j.jbc.2024.107881
Neuronal splicing of the unmethylated histone H3K4 reader, PHF21A, prevents excessive synaptogenesis
Abstract
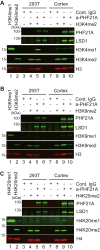
PHF21A is a histone-binding protein that recognizes unmethylated histone H3K4, the reaction product of LSD1 histone demethylase. PHF21A and LSD1 form a complex, and both undergo neuron-specific microexon splicing. The PHF21A neuronal microexon interferes with nucleosome binding, whereas the LSD1 neuronal microexon weakens H3K4 demethylation activity and can alter the substrate specificity to H3K9 or H4K20. However, the temporal expression patterns of PHF21A and LSD1 splicing isoforms during brain development and their biological roles remain unknown. In this work, we report that neuronal PHF21A isoform expression precedes neuronal LSD1 expression during human neuron differentiation and mouse brain development. The asynchronous splicing events resulted in stepwise deactivation of the LSD1-PHF21A complex in reversing H3K4 methylation. An unbiased proteomics survey revealed that the enzymatically inactive LSD1-PHF21A complex interacts with neuron-specific binding partners, including MYT1-family transcription factors and post-transcriptional mRNA processing proteins such as VIRMA. The interaction with the neuron-specific components, however, did not require the PHF21A microexon, indicating that the neuronal proteomic milieu, rather than the microexon-encoded PHF21A segment, is responsible for neuron-specific complex formation. Finally, by using two Phf21a mutant mouse models, we found that Phf21a neuronal splicing prevents excess synapse formation that otherwise would occur when canonical PHF21A is expressed in neurons. These results suggest that the role of the PHF21A microexon is to dampen LSD1-mediated H3K4 demethylation, thereby containing aberrant synaptogenesis.
Keywords: LSD1; PHF21A; histone demethylase; histone methylation; microexon splicing.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest M. C. W. is the CEO of Neurodigitech, LLC. The other authors declare that they have no conflicts of interest with the contents of this article.
Figures




Update of
-
Asynchronous microexon splicing of LSD1 and PHF21A during neurodevelopment.bioRxiv [Preprint]. 2024 Mar 21:2024.03.21.586181. doi: 10.1101/2024.03.21.586181. bioRxiv. 2024. Update in: J Biol Chem. 2024 Nov;300(11):107881. doi: 10.1016/j.jbc.2024.107881. PMID: 38562691 Free PMC article. Updated. Preprint.
References
-
- Najmabadi H., Hu H., Garshasbi M., Zemojtel T., Abedini S.S., Chen W., et al. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature. 2011;478:57–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

