Unraveling the roles and mechanisms of mitochondrial translation in normal and malignant hematopoiesis
- PMID: 39396039
- PMCID: PMC11470598
- DOI: 10.1186/s13045-024-01615-9
Unraveling the roles and mechanisms of mitochondrial translation in normal and malignant hematopoiesis
Abstract
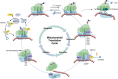
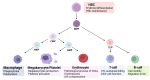
Due to spatial and genomic independence, mitochondria possess a translational mechanism distinct from that of cytoplasmic translation. Several regulators participate in the modulation of mitochondrial translation. Mitochondrial translation is coordinated with cytoplasmic translation through stress responses. Importantly, the inhibition of mitochondrial translation leads to the inhibition of cytoplasmic translation and metabolic disruption. Therefore, defects in mitochondrial translation are closely related to the functions of hematopoietic cells and various immune cells. Finally, the inhibition of mitochondrial translation is a potential therapeutic target for treating multiple hematologic malignancies. Collectively, more in-depth insights into mitochondrial translation not only facilitate our understanding of its functions in hematopoiesis, but also provide a basis for the discovery of new treatments for hematological malignancies and the modulation of immune cell function.
Keywords: HSC; Hematologic malignancy; Hematopoiesis; Immune cell; Mitochondrial translation; T cell.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Emerging insights into epigenetics and hematopoietic stem cell trafficking in age-related hematological malignancies.Stem Cell Res Ther. 2024 Nov 6;15(1):401. doi: 10.1186/s13287-024-04008-4. Stem Cell Res Ther. 2024. PMID: 39506818 Free PMC article. Review.
-
Autophagy and Metabolism in Normal and Malignant Hematopoiesis.Int J Mol Sci. 2021 Aug 9;22(16):8540. doi: 10.3390/ijms22168540. Int J Mol Sci. 2021. PMID: 34445246 Free PMC article. Review.
-
RNA-binding proteins in hematopoiesis and hematological malignancy.Blood. 2019 May 30;133(22):2365-2373. doi: 10.1182/blood-2018-10-839985. Epub 2019 Apr 9. Blood. 2019. PMID: 30967369 Free PMC article. Review.
-
Clonal hematopoiesis: Molecular basis and clinical relevance.Leuk Res. 2020 Nov;98:106457. doi: 10.1016/j.leukres.2020.106457. Epub 2020 Sep 23. Leuk Res. 2020. PMID: 33010619 Review.
-
Extracellular Vesicles and Hematopoietic Stem Cell Aging.Arterioscler Thromb Vasc Biol. 2021 Aug;41(8):e399-e416. doi: 10.1161/ATVBAHA.120.314643. Epub 2021 Jun 3. Arterioscler Thromb Vasc Biol. 2021. PMID: 34078091 Review.
Cited by
-
Mitochondrial ribosomal proteins: potential targets for cancer prognosis and therapy.Front Oncol. 2025 Apr 30;15:1586137. doi: 10.3389/fonc.2025.1586137. eCollection 2025. Front Oncol. 2025. PMID: 40371222 Free PMC article. Review.
References
-
- Zhang L, Ging NC, Komoda T, Hanada T, Suzuki T, Watanabe K. Antibiotic susceptibility of mammalian mitochondrial translation. FEBS Lett. 2005;579(28):6423–7. - PubMed
-
- Wang B, Shi D, Yang S, Lian Y, Li H, Cao M et al. Mitochondrial tRNA pseudouridylation governs erythropoiesis. Blood. 2024;blood.2023022004. - PubMed
-
- Lisci M, Barton PR, Randzavola LO, Ma CY, Marchingo JM, Cantrell DA, et al. Mitochondrial translation is required for sustained killing by cytotoxic T cells. Science. 2021;374(6565):eabe9977. - PubMed
Publication types
MeSH terms
Grants and funding
- 2022YFA1103500/National Key Research and Development Program of China
- 82222003/National Natural Science Foundation of China
- 82341206/National Natural Science Foundation of China
- 2024SSYS0024/Key Research and Development Program of Zhejiang Province
- 2024SSYS0025/Key Research and Development Program of Zhejiang Province
LinkOut - more resources
Full Text Sources

