Bridging the gap: Insights in the immunopathology of Lyme borreliosis
- PMID: 39396370
- PMCID: PMC11628917
- DOI: 10.1002/eji.202451063
Bridging the gap: Insights in the immunopathology of Lyme borreliosis
Abstract
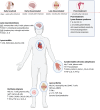
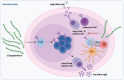
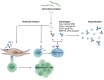
Lyme borreliosis (LB), caused by Borrelia burgdorferi sensu lato (Bbsl) genospecies transmitted by Ixodes spp. ticks, is a significant public health concern in the Northern Hemisphere. This review highlights the complex interplay between Bbsl infection and host-immune responses, impacting clinical manifestations and long-term immunity. Early localized disease is characterized by erythema migrans (EM), driven by T-helper 1 (Th1) responses and proinflammatory cytokines. Dissemination to the heart and CNS can lead to Lyme carditis and neuroborreliosis respectively, orchestrated by immune cell infiltration and chemokine dysregulation. More chronic manifestations, including acrodermatitis chronica atrophicans and Lyme arthritis, involve prolonged inflammation as well as the development of autoimmunity. In addition, dysregulated immune responses impair long-term immunity, with compromised B-cell memory and antibody responses. Experimental models and clinical studies underscore the role of Th1/Th2 balance, B-cell dysfunction, and autoimmunity in LB pathogenesis. Moreover, LB-associated autoimmunity parallels mechanisms observed in other infectious and autoimmune diseases. Understanding immune dysregulation in LB provides insights into disease heterogeneity and could provide new strategies for diagnosis and treatment.
Keywords: Autoimmunity; Borrelia burgdorferi; Immune dysregulation; Inflammation; Lyme borreliosis; Th1/Th2 Balance.
© 2024 The Author(s). European Journal of Immunology published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no commercial or financial conflict of interest.
Figures
References
-
- Piesman, J. and Gern, L. , Lyme borreliosis in Europe and North America. Parasitology. 2004;129 Suppl: S191–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

