Palmitoleate protects against lipopolysaccharide-induced inflammation and inflammasome activity
- PMID: 39396700
- PMCID: PMC11585775
- DOI: 10.1016/j.jlr.2024.100672
Palmitoleate protects against lipopolysaccharide-induced inflammation and inflammasome activity
Abstract
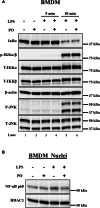
Inflammation is part of natural immune defense mechanism against any form of infection or injury. However, prolonged inflammation could perturb cell homeostasis and contribute to the development of metabolic and inflammatory diseases, including maternal obesity, diabetes, cardiovascular diseases, and metabolic dysfunction-associated steatotic liver diseases (MASLD). Polyunsaturated fatty acids have been shown to mitigate inflammatory response by generating specialized proresolving lipid mediators, which take part in resolution of inflammation. Similarly here, we show that palmitoleate, an omega-7 monounsaturated fatty acid exerts anti-inflammatory properties in response to lipopolysaccharide (LPS)-mediated inflammation. Exposure of bone marrow-derived macrophages (BMDMs) to LPS or TNFα induces robust increase in the expression of proinflammatory cytokines and supplementation of palmitoleate inhibited LPS-mediated upregulation of proinflammatory cytokines. We also observed that palmitoleate was able to block LPS + ATP-induced inflammasome activation mediated cleavage of procaspase 1 and prointerleukin-1β. Further, treatment of palmitoleate protects against LPS-induced inflammation in human THP-1-derived macrophages and trophoblasts. Coexposure of LPS and palmitate (saturated free fatty acid) induces inflammasome and cell death in BMDMs, however, treatment of palmitoleate blocked LPS and palmitate-induced cell death in BMDMs. Further, LPS and palmitate together results in the activation of mitogen-activated protein kinases and pretreatment of palmitoleate inhibited the activation of mitogen-activated protein kinases and nuclear translocation of nuclear factor kappa B in BMDMs. In conclusion, palmitoleate shows anti-inflammatory properties against LPS-induced inflammation and LPS + palmitate/ATP-induced inflammasome activity and cell death.
Keywords: macrophages; mitogen-activated protein kinase; monounsaturated fatty acids; obesity; placenta; pregnancy; trophoblasts.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures







References
-
- Meizlish M.L., Franklin R.A., Zhou X., Medzhitov R. Tissue homeostasis and inflammation. Annu. Rev. Immunol. 2021;39:557–581. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

