Cost-effectiveness analysis of the geko™ device (an NMES technology) in managing venous leg ulcers in UK healthcare settings
- PMID: 39396902
- PMCID: PMC11471310
- DOI: 10.1111/iwj.70048
Cost-effectiveness analysis of the geko™ device (an NMES technology) in managing venous leg ulcers in UK healthcare settings
Abstract
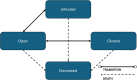
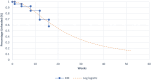
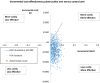
This study evaluates the cost-effectiveness of the geko device a neuromuscular electro-stimulator technology with standard of care (SoC) versus SoC alone for venous leg ulcer (VLU) treatment, from the UK National Health Service (NHS) perspective over 12 months. Research was conducted across NHS UK facilities, primarily within community services and outpatient leg ulcer clinics, encompassing a total of 51 patients. A partitioned survival model, based on a two-arm randomised controlled trial, assessed wound healing rates using Kaplan-Meier curves and parametric extrapolations over a 12-month time horizon. Costs were derived from UK reference costs the British National Formulary, and the Personal Social Services Research Unit (2021/22). The primary outcome measured was the incremental cost per quality-adjusted life-year gained. The geko device provides additional benefits by stimulating the lateral popliteal nerve, augmenting venous, arterial, and microvascular flow. The addition of the geko device to SoC significantly enhanced outcomes, increasing healing probability by 68% compared to SoC. This integration would result in a cost saving of £774.14 per patient when compared to the SoC alone across the NHS. Economic analyses indicate that integrating the geko device into SoC protocols would reduce the overall NHS expenditure on VLU wound management by as much as 15%. The approach also positively impacted health-related quality of life. The geko™ device when used adjunctively with SoC would be a cost-effective method for managing chronic VLUs within the NHS, improving healing rates and offering economic benefits.
Keywords: NMES; cost saving; cost‐effectiveness; venous leg ulcers; wound management.
© 2024 The Author(s). International Wound Journal published by Medicalhelplines.com Inc and John Wiley & Sons Ltd.
Conflict of interest statement
KH and RT have received investigator grants, honoraria, and consulting fees from several medical device companies, including Firstkind Ltd.
Figures



References
-
- NHS Scotland . NHS INform Scotland. Accessed April 14, 2023. https://www.nhsinform.scot/illnesses‐and‐conditions/skin‐hair‐and‐nails/....
-
- NHS England. 2020. Accessed April 13, 2023. https://www.england.nhs.uk/long‐read/providing‐proactive‐care‐for‐people...
-
- NNWCS Programme . NWCSP National Wound Care Strategy Programme. Accessed November 20, 2023. https://www.nationalwoundcarestrategy.net/wp‐content/uploads/2023/08/NWC...
-
- NHS England. 2023. Accessed April 13, 2023. https://www.longtermplan.nhs.uk/online-version/overview-and-summary/
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

